
Budenofalk

Ask a doctor about a prescription for Budenofalk

How to use Budenofalk
Leaflet attached to the packaging: information for the user
Budenofalk, 2 mg/dose, rectal foam
Budesonide
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Budenofalk and what is it used for
- 2. Important information before using Budenofalk
- 3. How to use Budenofalk
- 4. Possible side effects
- 5. How to store Budenofalk
- 6. Contents of the packaging and other information
1. What is Budenofalk and what is it used for
Budenofalk in the form of rectal foam contains budesonide as the active substance. This medicine belongs to a group of locally acting steroids used to treat inflammatory bowel diseases. It is indicated for the treatment of active, ulcerative proctitis, limited to the rectum and sigmoid colon.
2. Important information before using Budenofalk
When not to use Budenofalk, rectal foam:
- if the patient is allergicto budesonide or any of the other ingredients of this medicine (listed in section 6);
- if the patient has severe liver disease(liver cirrhosis).
Warnings and precautions
Before starting treatment with Budenofalk, rectal foam, the patient should discuss with their doctor if they have:
- tuberculosis;
- high blood pressure;
- diabetes or a family history of diabetes;
- bone fragility (osteoporosis);
- stomach or duodenal ulcer disease;
- increased eye pressure (glaucoma) or eye problems, such as cataracts;
- severe liver problems.
Typical side effects of corticosteroids may occur during treatment with Budenofalk, rectal foam, which can affect all parts of the body, especially when using the medicine in high doses and for a long time (see section 4. Possible side effects).
Additional precautions during treatment with Budenofalk, rectal foam
- The patient should inform their doctor if they have an infection. Symptoms of some infections may be atypical or less severe.
- If the patient has not had chickenpox or shingles, they should avoid contact with people who have these diseases. These diseases can be very severe. If the patient comes into contact with people who have chickenpox or shingles, they should see a doctor immediately.
- If the patient has not had measles, they should inform their doctor.
- If the patient is scheduled to be vaccinated, they should discuss it with their doctor first.
- If the patient is scheduled for surgery, they should inform their doctor about using Budenofalk, rectal foam.
- If the patient was previously treated with a stronger corticosteroid, they may experience a relapse of symptoms after switching to Budenofalk, rectal foam. In such a case, they should contact their doctor.
- If the patient experiences blurred vision or other vision disturbances, they should contact their doctor.
Using Budenofalk, rectal foam, may result in positive doping test results.
Budenofalk, rectal foam, and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take. In particular:
- cardiac glycosides, such as digoxin (medicines used to treat heart diseases);
- diuretics(medicines used to remove excess fluid from the body);
- ketokonazole or itraconazole(medicines used to treat fungal infections);
- antibiotics(medicines used to treat infections, such as clarithromycin);
- carbamazepine(a medicine used to treat epilepsy);
- rifampicin(a medicine used to treat tuberculosis);
- estrogens or oral contraceptives.
Some medicines may enhance the effect of Budenofalk, rectal foam, and the doctor may want to closely monitor the patient's condition when taking such medicines (including some HIV medicines: ritonavir, cobicistat). Budenofalk, rectal foam, may affect the results of tests performed by the doctor or in the hospital. Before undergoing any tests, the patient should tell their doctor about using Budenofalk, rectal foam.
Budenofalk, rectal foam, with food and drink
The patient should not drink grapefruit juicewhile using this medicine, as it may change its effect.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine. The patient should use Budenofalk, rectal foam, during pregnancy only if recommended by their doctor. Budesonide passes into breast milk in small amounts. If the patient is breastfeeding, they should use Budenofalk, rectal foam, only if recommended by their doctor.
Driving and using machines
No effects of the medicine on driving or using machines have been observed.
Budenofalk, rectal foam, contains propylene glycol, cetyl alcohol, and cetostearyl alcohol.
This medicine contains 600.3 mg of propylene glycol in each rectal foam spray of Budenofalk, 2 mg/dose. Propylene glycol may cause skin irritation. Cetyl alcohol and cetostearyl alcohol (emulsifying wax component) may cause local skin reactions (e.g., contact dermatitis).
3. How to use Budenofalk, rectal foam
This medicine should always be used as directed by the doctor. In case of doubts, the patient should consult their doctor or pharmacist.
Recommended dose:
Adults should use 1 dose per day, which corresponds to 2 mg of budesonide. The use of Budenofalk in children is not recommended due to insufficient data on safety.
Method of administration:
The medicine is intended for rectal use only and should be inserted through the anus. It is not intended for oral use and should not be swallowed. Budenofalk can be administered in the morning or evening. Best results are achieved when the medicine is used after bowel movements.
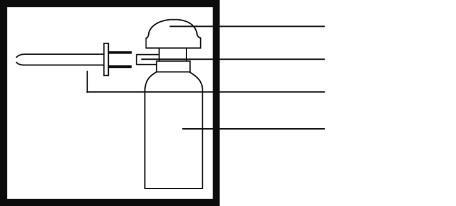
pump dome
nozzle
applicator
aerosol container
The applicators are in a special stand. The stand should be held firmly and the applicator pulled out with a decisive motion.
Instructions for use:
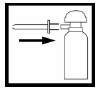
Press the applicator firmly onto the nozzle of the aerosol container. Shake the aerosol container for about 15 seconds to mix the contents.
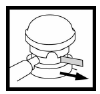
Before first use, remove the protective ring from under the pump dome.
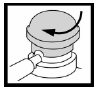
Turn the pump dome on the aerosol container until the semicircular notch is in line with the applicator. The aerosol container is now ready for use.
Administration of the foam:
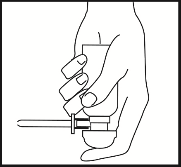
Place the index finger on the pump dome and turn the aerosol container upside down. The aerosol container will only work properly when held with the pump dome facing downwards, in a position as close to vertical as possible. Stand with one foot on a stool or chair or lie on their side, with the lower leg straight and the upper leg bent to maintain balance. Insert the applicator into the rectum as far as possible without discomfort. Press the pump dome once, fully, and then release it slowly – after releasing the dome, the foam will be dispensed from the aerosol container. Hold the applicator in the same position for 10-15 seconds before removing it from the rectum. This will ensure that the full dose is administered to the rectum and help prevent the foam from leaking out.
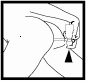
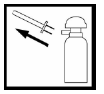
After using the foam, remove the applicator and dispose of it with household waste, using the provided plastic bag. Use a new applicator for the next dose. To prevent accidental foam release from the aerosol container between doses, turn the pump dome so that the semicircular notch is on the opposite side from the nozzle.
Duration of therapy:
The duration of treatment will be determined by the doctor. Usually, the acute inflammatory condition subsides after 6 to 8 weeks. The doctor will then recommend stopping the use of Budenofalk. If the patient feels that the effect of Budenofalk is too strong or too weak, they should consult their doctor.
Using a higher than recommended dose of Budenofalk, rectal foam
In case of a single dose that is too high, the next dose should be taken as recommended by the doctor. Do not take a lower dose. In case of doubts, consult a doctor, who will decide on further action. Take the carton and this leaflet with you if possible.
Missing a dose of Budenofalk, rectal foam
If a dose is missed, continue treatment with the prescribed dose. Do not take a double dose to make up for the missed dose.
Stopping the use of Budenofalk, rectal foam
Consult a doctor before deciding to stop or prematurely end treatment. Do not stop using the medicine abruptly, as this may worsen the patient's condition. Continue using the medicine, even if the patient feels better, until the doctor informs them that they can stop treatment. In case of any further doubts about using this medicine, consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If the patient experiences any of the following symptoms after taking this medicine, they should contact their doctor immediately:
- infection;
- headache;
- changes in behavior, such as depression, irritability, euphoria, nervousness, anxiety, or aggression.
The following side effects have also been reported:
Common: may affect up to 1 in 10 people
- burning or pain in the rectum;
- Cushing's syndrome: e.g., rounded face, obesity in the trunk, decreased glucose tolerance, high blood sugar, high blood pressure, fluid retention in tissues (e.g., swelling of the legs), increased potassium excretion (hypokalemia), irregular menstrual periods in women, excessive hair growth in women, impotence, abnormal laboratory test results (decreased adrenal function), red lines on the skin (striae), acne;
- indigestion, stomach upset;
- increased risk of infections;
- muscle and joint pain, muscle weakness, muscle tremors;
- fragile bones (osteoporosis);
- headache;
- mood changes, such as depression, irritability, and euphoria;
- skin rash due to hypersensitivity, red spots caused by bleeding under the skin, delayed wound healing, local skin reactions, such as contact dermatitis.
Uncommon: may affect up to 1 in 100 people
- increased appetite;
- changes in blood test results (elevated erythrocyte sedimentation rate - ESR, increased white blood cell count);
- nausea, stomach pain, bloating, abdominal tingling or numbness, anal fissure, mouth ulcers, frequent need to empty the bowels, rectal bleeding;
- stomach or duodenal ulcers;
- changes in liver function tests;
- changes in pancreatic function, changes related to adrenal hormones;
- urinary tract infections;
- dizziness, smell disturbances;
- insomnia, nervousness with excessive physical activity, anxiety;
- excessive sweating, weakness;
- weight gain.
Rare: may affect up to 1 in 1,000 people
- glaucoma;
- cataract;
- blurred vision;
- pancreatitis;
- bone tissue loss due to poor blood circulation (bone necrosis);
- aggression;
- bruises.
Very rare: may affect up to 1 in 10,000 people
- growth retardation in children;
- constipation;
- increased intracranial pressure, also with increased eye pressure (papilledema) in young people;
- increased risk of blood clots, vasculitis (related to discontinuation of corticosteroids after long-term treatment);
- fatigue, general feeling of being unwell.
The above side effects are typical of steroid medicines and can also be expected to occur with other steroids. The occurrence of the above side effects depends on the doses used, the duration of treatment, concurrent or previous treatment with other corticosteroid-containing medicines, and the individual patient's sensitivity. Some of the above side effects have been reported only after long-term oral use of budesonide. The risk of side effects after using Budenofalk, rectal foam, is generally lower than with systemically acting corticosteroids (affecting the whole body), due to the local action of the medicine. If the patient was previously treated with a stronger corticosteroid, they may experience a relapse of symptoms after switching to Budenofalk, rectal foam.
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, more information can be collected on the safety of this medicine.
5. How to store Budenofalk
The medicine should be stored out of sight and reach of children. Do not use this medicine after the expiry date stated on the carton and on the bottom of the container. The expiry date refers to the last day of the month. Store in a temperature below 25°C. Do not freeze. The container is under pressure and contains 6.5% of a flammable propellant gas. Store away from fire or sparks, including cigarettes. Protect the container from direct sunlight and do not open it by force, pierce, or burn it, even after it is empty. Do not spray near fire or hot surfaces. The contents of the container should be used within 4 weeks of first opening. Medicines should not be disposed of via wastewater or household waste. Ask a pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Budenofalk contains
- The active substance of Budenofalk is budesonide. Each rectal foam dose contains 2 mg of budesonide.
- The other excipients are: Emulsion: propylene glycol, purified water, emulsifying wax, macrogol stearate, cetyl alcohol, citric acid monohydrate, disodium edetate.
Propellant gas: propane/n-butane/isobutane, nitrogen.
What Budenofalk looks like and what the pack contains
Budenofalk is a white or almost white, firm foam with a creamy consistency. Budenofalk is available in packs (carton) containing 1 aerosol container, 14 applicators, and 14 plastic bags, which are used for hygienic disposal of used applicators. One aerosol container should be sufficient for at least 14 doses of 1.3 g of rectal foam – corresponding to 14 administrations of the medicine.
Marketing authorization holder and importer
Dr. Falk Pharma GmbH, Leinenweberstrasse 5, 79108 Freiburg, Germany. For more information, contact the representative of the marketing authorization holder in Poland: Ewopharma AG Sp. z o.o., ul. Leszno 14, 01-192 Warsaw, tel. 22 620 11 71.
Date of last revision of the leaflet: 11/2021
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterDr. Falk Pharma GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BudenofalkDosage form: Tablets, 9 mgActive substance: budesonideManufacturer: Cosmo S.p.A.Prescription requiredDosage form: Capsules, 3 mgActive substance: budesonideManufacturer: Astrea FontainePrescription requiredDosage form: Capsules, 3 mgActive substance: budesonideManufacturer: Laboratorios Liconsa S.A.Prescription required
Alternatives to Budenofalk in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Budenofalk in Испания
Alternative to Budenofalk in Украина
Online doctors for Budenofalk
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Budenofalk – subject to medical assessment and local rules.














