ENTOCORD 2 mg TABLETS AND SOLUTION FOR RECTAL SUSPENSION

How to use ENTOCORD 2 mg TABLETS AND SOLUTION FOR RECTAL SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Entocord 2 mg tablet and solvent for rectal suspension
budesonide
Read this package leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the package leaflet:
- What is Entocord and what is it used for
- What you need to know before you use Entocord
- How to use Entocord
- Possible side effects
- Storage of Entocord
- Contents of the pack and further information
1. What is Entocord and what is it used for
Entocord (budesonide) belongs to a group of medicines called glucocorticosteroids (a type of cortisone) that are used to reduce inflammation.
Entocord is used in the treatment of ulcerative colitis, a disease caused by inflammation of the intestinal wall, affecting the rectum and the sigmoid and descending colon.
2. What you need to know before you use Entocord
Do not use Entocord
If you are allergic to budesonide or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using Entocord.
You must always inform your doctor of the following situations:
- If you have other health problems, such as liver disease.
- If you are suffering from or contract any infection, particularly chickenpox or measles.
- If you have diabetes (including family history), brittle bones (osteoporosis), stomach ulcers, or high blood pressure.
- If you have any eye disease such as glaucoma (including family history) or cataracts.
- If your symptoms worsen while you are using Entocord.
Do not stop treatment with Entocord until your doctor tells you to.
Contact your doctor if you experience blurred vision or other visual disturbances.
Entocord has been prescribed specifically for your current illness. Do not use it for other problems unless your doctor tells you to.
If you were previously treated with "cortisone" tablets (such as prednisone, prednisolone, or methylprednisolone) and your medication has been changed to Entocord, symptoms that may have bothered you before may temporarily reappear, such as skin rashes, muscle and joint pain. If any of these symptoms bother you or symptoms such as headache, fatigue, nausea, or vomiting appear, please contact your doctor.
Children and adolescents
There is no long-term data on treatments in children and adolescents; it is recommended to regularly check their height.
Other medicines and Entocord
Tell your doctor, pharmacist, or nurse if you are taking, have recently taken, or might take any other medicines.
Certain medicines may interact with Entocord; in these cases, it may be necessary to change the dose or stop treatment with one of the medicines.
It is important that you inform your doctor if you are taking any of the following medications: ketoconazole, itraconazole (medicines used against fungal infections), carbamazepine (antiepileptic) or, in women, estrogens, and some contraceptives.
Some medicines may increase the effects of Entocord, and your doctor may want to monitor you closely if you are taking these medicines (including some medicines for the treatment of HIV: ritonavir (and other HIV protease inhibitors, cobicistat).
Diagnostic tests for pituitary gland activity may show false low results due to adrenal function suppression.
Pregnancy and breastfeedingIf you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor, pharmacist, or nurse before using this medicine.
Driving and using machinesEntocord does not affect the ability to drive or use machines.
Entocord contains methylparaben and propylparaben
May cause allergic reactions (possibly delayed) because it contains methylparaben and propylparaben.
If you are an athlete, you should be aware that this medicine contains a component that may result in a positive doping test.
3. How to use Entocord
Follow the instructions for administration of this medicine exactly as indicated by your doctor, pharmacist, or nurse. In case of doubt, consult your doctor, pharmacist, or nurse again.
Remember to use your medicine.
Your doctor will indicate the duration of your treatment with Entocord. Do not stop treatment before your doctor tells you to.
Before using Entocord for the first time, it is important that you read the Instructions for Use described below. These instructions will indicate how to prepare and use Entocord. Follow the instructions carefully.
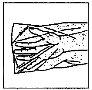
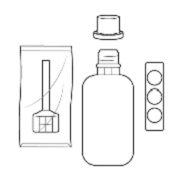
Instructions for use/manipulation Entocord should be administered at night before going to bed. Entocord consists of: a tablet, a 115 mL solvent bottle, and a rectal cannula individually packaged. For reconstitution of the medicine, the tablet must be dissolved in the solvent before use. To correctly administer Entocord, follow the instructions carefully: |
| |
How to prepare Entocord | ||
|
| |
|
| |
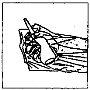
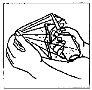
How to administer Entocord Plastic covers are included in the package to protect your hand during administration of the medicine (see the illustrations later).
Adopt a comfortable position for sleeping. Try to retain Entocord for as long as possible, preferably all night. |
| |
|
|
|
|
|
|
Once the rectal suspension is prepared, it must be administered immediately. It should not be stored in the bottle.
The dosage is adapted to each individual. Follow your doctor's instructions carefully. These instructions may be different from the information contained in this package leaflet.
Recommended dose for adults
It is recommended to administer one Entocord (tablet + 115 mL solvent bottle) per day (every night) for four weeks. The best time to use Entocord is at night, just before going to bed. This way, Entocord will remain in your intestine for as long as possible while you are sleeping.
The full effect is usually achieved within 2-4 weeks. However, if your symptoms have not improved after four weeks of treatment, your doctor may extend your treatment for another 4 weeks.
Entocord should be used regularly as prescribed. Do not forget to administer Entocord even if you start to feel better.
Elderly patients
The same dosage as for adults.
If you use more Entocord than you should
In case of overdose or accidental ingestion, consult the Toxicology Information Service. Telephone 91 562 04 20
If you forget to use Entocord
If you occasionally forget to administer a dose of Entocord, it is not necessary to make up for the missed dose; simply continue with the next dose as prescribed.
Do not use a double dose to make up for missed doses.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you have an allergic reaction, consult your doctor immediately.The signs may include skin lumps (hives) or swelling of the face, lips, mouth, tongue, or throat. This can make it difficult to breathe.
Other possible side effects:
Common: may affect up to 1 in 10 people
- Stomach or intestine pain, such as stomach pain, flatulence, diarrhea, heartburn, and feeling unwell.
- Cramps.
- Skin reactions, such as rash and skin eruption.
- Changes in behavior, such as nervousness, insomnia, mood changes, and depression.
- Palpitations.
- Low potassium levels in the blood.
- Menstrual disorders.
- Cushingoid features such as rounded face, acne, weight gain, and easy bruising.
Uncommon: may affect up to 1 in 100 people
- Involuntary movements or extreme restlessness, possibly accompanied by muscle spasms or twitches.
- Anxiety.
- Tremor.
Rare: may affect up to 1 in 1,000 people
- Disorder of the adrenal gland (a small gland near the kidney).
- Aggression.
- Opacity of the natural lens of the eye, including the posterior part (cataracts).
- Glaucoma (increased eye pressure).
- Blurred vision.
- Change in skin color due to internal bleeding.
Very rare: may affect up to 1 in 10,000 people
- Severe allergic reaction (called anaphylaxis) that can cause difficulty breathing or shock.
- Delayed growth rate in children and adolescents.
Frequency not known: cannot be estimated from the available data
- Allergic reactions that can cause swelling of the face, especially the eyelids, lips, tongue, or throat (angioedema).
Medicines like Entocord (corticosteroids) can affect the normal production of steroid hormones in your body. The effects include:
- Changes in bone mineral density (thinning of the bones).
- Glaucoma (increased eye pressure).
- Decreased growth rate in children and adolescents.
- Disorder of the adrenal gland (a small gland near the kidney).
Most of the side effects mentioned in this list can also be expected with treatment with other glucocorticoids.
Do not be alarmed by this list of side effects. You may not get any of them. If you think any of the side effects you are experiencing are serious or if you notice any side effects not mentioned in this package leaflet, tell your doctor, pharmacist, or nurse.
Reporting of side effects:
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this package leaflet. You can also report side effects directly through the Spanish Medicines Monitoring System: https://www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Entocord
Keep this medicine out of the sight and reach of children. Do not store the tablets and solvent bottles at a temperature above 30°C.
Do not use this medicine after the expiry date which is stated on the packaging after EXP. The expiry date is the last day of the month shown.
Medicines should not be disposed of via wastewater or household waste. Return the packaging and any unused medicine to a pharmacy. Ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and further information
Composition of Entocord
- The active substance is budesonide. Entocord (tablet and 115 mL solvent bottle) contains 2 mg of budesonide per 100 mL (0.02 mg/mL).
- Entocord consists of: a dispersible tablet, a 115 mL solvent bottle, and a rectal cannula individually packaged.
- Each tablet contains: 2.3 mg of the active substance budesonide
- The other excipients are: lactose, colorant [riboflavin (E101)], crospovidone, colloidal anhydrous silica, and magnesium stearate.
- Each 115 mL solvent bottle contains: sodium chloride, preservatives [methylparaben (E218), propylparaben (E216)], and water.
Appearance of the product and contents of the pack
Entocord is presented as dispersible tablets and solvent for rectal suspension. The packs contain a blister pack with 7 tablets, 7 solvent bottles of 115 mL, 7 rectal cannulas (enema applicators), and 7 plastic covers to use during enema application.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Tillotts Pharma GmbH
Warmbacher Str. 80
79618 Rheinfelden
Germany
Manufacturer:
Lusomedicamenta, Sociedade Técnica Farmacêutica, SA
Estrada Consiglieri Pedroso, 69-B
Queluz de Baixo
2730-055 Barcarena
Portugal
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Tillotts Pharma Spain, S.L.U.
Travessera de Gràcia 58, 5º 3ª
08006 Barcelona
Spain
Date of the last revision of this package leaflet:July 2023
Other sources of information
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price40.31 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ENTOCORD 2 mg TABLETS AND SOLUTION FOR RECTAL SUSPENSIONDosage form: MODIFIED-RELEASE CAPSULE, 3 mg budesonideActive substance: budesonideManufacturer: Tillotts Pharma GmbhPrescription requiredDosage form: RECTAL SEMISOLID, 2 mgActive substance: budesonideManufacturer: Dr. Falk Pharma GmbhPrescription requiredDosage form: CAPSULE, 3 mg budesonideActive substance: budesonideManufacturer: Dr. Falk Pharma GmbhPrescription required
Online doctors for ENTOCORD 2 mg TABLETS AND SOLUTION FOR RECTAL SUSPENSION
Discuss questions about ENTOCORD 2 mg TABLETS AND SOLUTION FOR RECTAL SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions




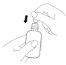 (Fig. 1).
(Fig. 1).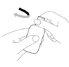 (Fig. 2).
(Fig. 2).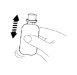 (Fig. 3).
(Fig. 3).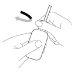 (Fig. 4).
(Fig. 4).