
INNOHEP 3,500 IU Anti-Xa/0.35 mL Injectable Solution in Syringes

How to use INNOHEP 3,500 IU Anti-Xa/0.35 mL Injectable Solution in Syringes
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
innohep 3,500 IU anti-Xa/0.35 ml solution for injection in pre-filled syringes
(10,000 IU anti-Xa/ml)
tinzaparin sodium
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
|
Contents of the pack
- What is innohep and what it is used for
- What you need to know before you use innohep
- How to use innohep
- Possible side effects
- Storage of innohep
- Contents of the pack and other information
1. What is innohep and what it is used for
innohep is a medicine that makes the blood more fluid by reducing the blood's natural ability to form clots.
innohep is used to
- prevent the formation of blood clots in adults before and after surgery.
- prevent the formation of blood clots in adults with a higher risk of blood clot formation, for example, due to an acute illness with limited mobility.
prevent the formation of blood clots in the blood in hemodialysis equipment in patients undergoing hemodialysis or hemofiltration. In hemodialysis, waste and fluid from the blood are removed by the machine and the dialysis filter that acts like an artificial kidney.
2. What you need to know before you use innohep
Do not use innohep:
- if you are allergic to tinzaparin sodium or any of the other ingredients of this medicine (listed in section 6).
- if you have or have had heparin-induced thrombocytopenia.
- if you have a severe bleeding disorder (e.g., in the brain, spinal cord, eyes, or stomach).
- if you have a severe infection in the heart (septic endocarditis).
Warnings and precautions
Consult your doctor or nurse before starting to use innohep:
- if you are going to have a spinal or epidural anesthesia or a lumbar puncture.
- if you know you have a tendency to bleed.
- if you are being treated with other medicines by injection into a muscle.
- if you have a low platelet count in your blood.
- if you have high potassium levels in your blood (hyperkalemia).
- if you have a prosthetic heart valve.
- if you have kidney problems.
Children and adolescents
innohep is not indicated for use in children and adolescents.
Using innohep with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines. Some medicines may interact with the effect of innohep.
Tell your doctor if you are using any of the following medicines as you may bleed more easily:
- Medicines for the treatment of inflammation and pain, especially non-steroidal anti-inflammatory drugs (NSAIDs), such as acetylsalicylic acid.
- Medicines used to dissolve blood clots (thrombolytic agents)
- Medicines that block the action of vitamin K (vitamin K antagonists)
- Activated protein C
- Direct inhibitors of factor Xa and IIa.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
innohep in pre-filled syringes can be used during all trimesters of pregnancy.
If epidural anesthesia is required, you must inform your doctor that you are using innohep.
Tell your doctor or nurse if you have a prosthetic heart valve.
Driving and using machines
innohep does not affect the ability to drive and use machines.
innohep contains sodium
This medicine contains less than 23 mg of sodium (1 mmol) per ml; it is essentially “sodium-free”.
3. How to use innohep
Follow exactly the administration instructions of this medicine indicated by your doctor or nurse. If in doubt, consult your doctor or nurse again.
Your doctor may request that you have routine blood tests to assess the effect of innohep.
To prevent the formation of blood clots in your veins
innohep must be injected under the skin (subcutaneous injection). The dose and duration of treatment will depend on the type of surgery or illness you have. Your doctor will indicate the suitable dose for you and the duration of your treatment.
To prevent the formation of blood clots in the blood during hemodialysis or hemofiltration
innohep will be introduced into the circuits of the hemodialysis machine or administered into a vein. The dose will depend on the duration of dialysis.
Instructions on how to inject innohep:
- Wash your hands carefully before injecting this medicine. Clean the skin around the injection site with an alcohol swab and let it dry without rubbing.
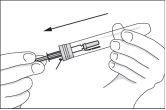
- Open the syringe container by lifting the colored cap all the way back and remove the syringe.
Inspect the contents of the syringe before use. If you notice turbidity or a precipitate in the medicine, do not use it and take another syringe. The medicine may have a yellowish color during storage, but it can be used as long as the solution is clear and the expiration date has not been exceeded. Each syringe can only be used once.
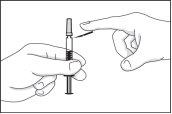
- Fold the safety device downwards, separating it from the needle protector cap.
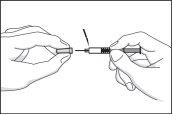
- Remove the cap that protects the needle without twisting it. Do not pull the plunger back or remove the air bubble contained in the syringe. If the air bubble is not correctly positioned next to the plunger, gently tap the syringe until the air bubble is in place next to the plunger.
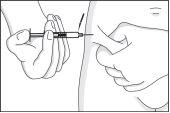
- Take a skin fold, without pressing, between the thumb and index finger of one hand, and with the other hand, insert the needle vertically into the skin fold, i.e., at a right angle to the skin.
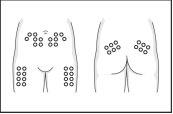
- Slowly inject the necessary dose into the fatty tissue, for example, the abdominal skin, the front of the thighs, the lower back, or the back of the arms. Wait a few seconds to allow the solution to spread before removing the needle and releasing the skin fold.
- Clean the area with a swab if you have bled. Choose a different injection site for the next time (e.g., alternating between the left and right side of the abdomen).
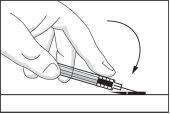
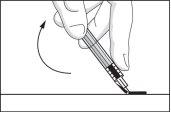
- Fold the safety device back to its original position so that it is parallel to the needle. Then, with the safety device flat on a rigid surface, press down until the needle is locked into the device.
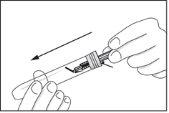
- The used syringe, with the needle facing down, can be inserted into the syringe container or into a container for sharp objects. This way, the syringe is safe, and the container with the syringe or the container for sharp objects can be delivered to a hospital or pharmacist for destruction.
If you use more innohep than you should
If you are treated with more innohep than you need, bleeding may occur. Consult your doctor or nurse immediately if you do not feel well or think you have been given too high a dose of innohep.
In case of overdose or accidental ingestion, you can also contact the Toxicology Information Service. Phone 91 562 04 20.
If you forget to use innohep
Do not take a double dose to make up for forgotten doses. If you have forgotten to take more than one dose, consult your doctor as soon as possible.
If you stop using innohep
If you stop using innohep, the effect of making the blood more fluid will cease. Do not stop using innohep without consulting your doctor or nurse.
If you have any further questions on the use of this medicine, ask your doctor or nurse.
4. Possible side effects
Like all medicines, innohep can cause side effects, although not everybody gets them.
Serious side effects:
Rarely, serious side effects are observed that require immediate medical attention during treatment with innohep. If you experience any of the following symptoms, consult your doctor immediately or go to the nearest hospital for emergency treatment.
- Severe allergic reaction. Symptoms include sudden appearance of a severe skin rash, swelling of the throat, face, lips, or mouth, difficulty breathing.
- Severe bleeding. Symptoms include urine that is red or brown, stools that look like tar, unusual bruising (very painful, extensive, or dark) and any bleeding that does not stop.
The following side effects have been observed with the administration of innohep:
Common: may affect up to 1 in 10 people
- Bleeding. This can lead to complications such as anemia (low number of red blood cells) or bruising.
- Reactions at the injection site (including bruising, bleeding, pain, itching, redness, inflammation, and hardening of the injection site)
Uncommon: may affect up to 1 in 100 people
- Thrombocytopenia (decrease in the number of platelets in the blood)
- Hypersensitivity (allergic reaction)
- Bruising and skin discoloration
- Elevation of liver enzyme levels
- Dermatitis (skin inflammation)
- Rash and itching
Rare: may affect up to 1 in 1,000 people
- Heparin-induced thrombocytopenia (decrease in the number of platelets in the blood due to heparin treatment)
- Thrombocytosis (increase in the number of platelets in the blood)
- Angioedema (swelling of the face, lips, and tongue)
- Anaphylactic reaction (see above “Severe allergic reaction”)
- Hyperkalemia (elevated potassium levels in the blood)
- Toxic skin rash
- Necrosis of the skin
- Hives
- Osteoporosis, observed in long-term treatments
- Priapism (prolonged, often painful, erection without previous sexual stimulation)
Pediatric population
Limited information is available, derived from a clinical trial and post-marketing data, indicating that the profile of adverse reactions in children and adolescents is comparable to that observed in adults.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines and Healthcare Products Agency (AEMPS) https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of innohep
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the carton after EXP. The expiry date is the last day of the month shown.
- No special storage conditions are required.
- Do not use this medicine if you notice turbidity or a precipitate in the syringe.
- The solution may have a yellowish color during storage, but this does not affect the quality of the product, which is still safe for use.
- Medicines should not be disposed of via wastewater or household waste. Place the containers and medicines you no longer need in the SIGRE point at the pharmacy. If in doubt, ask your pharmacist how to dispose of containers and medicines no longer needed. This will help protect the environment.
6. Contents of the pack and other information
Composition of innohep
- The active substance is tinzaparin sodium. Each ml of solution for injection contains 10,000 IU anti-Xa of tinzaparin sodium.
- The other ingredients are sodium acetate, sodium hydroxide, and water for injections.
Appearance and packaging
Solution for injection.
Transparent glass syringes containing a clear or pale yellow solution that does not show turbidity or sediment when the syringe is left to stand.
Pack sizes:
- 0.35 ml (3,500 IU anti-Xa), packs of 2, 10, and 50 syringes.
Not all pack sizes may be marketed.
Marketing authorisation holder
LEO Pharma A/S
Industriparken 55
DK-2750 Ballerup
Denmark
Manufacturer
Laboratoires LEO S.A.
39 Route de Chartres
FR-28500 Vernouillet Cedex
France
Date of last revision of this leaflet: December 2019
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) http://www.aemps.gob.es/.
- Country of registration
- Average pharmacy price37.15 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to INNOHEP 3,500 IU Anti-Xa/0.35 mL Injectable Solution in SyringesDosage form: INJECTABLE, 10000 IUActive substance: tinzaparinManufacturer: Leo Pharma A/SPrescription requiredDosage form: INJECTABLE, 12,000 IU anti-XaActive substance: tinzaparinManufacturer: Leo Pharma A/SPrescription requiredDosage form: INJECTABLE, 14,000 IUActive substance: tinzaparinManufacturer: Leo Pharma A/SPrescription required
Online doctors for INNOHEP 3,500 IU Anti-Xa/0.35 mL Injectable Solution in Syringes
Discuss questions about INNOHEP 3,500 IU Anti-Xa/0.35 mL Injectable Solution in Syringes, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















