
INASPIR ACCUHALER 50 micrograms/inhalation, POWDER FOR INHALATION

How to use INASPIR ACCUHALER 50 micrograms/inhalation, POWDER FOR INHALATION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is Inaspir Accuhaler and what is it used for
- What you need to know before you use Inaspir Accuhaler
- If you experience any of the above situations, do not increase the number of applications of Inaspir Accuhaler (your respiratory condition may worsen and you may become seriously ill). Go to your doctor as you may need to have your medication changed for the treatment of your asthma.
- Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
- How to use Inaspir Accuhaler
- Possible side effects
- Storing Inaspir Accuhaler
- Contents of the pack and further information
Introduction
Package Leaflet: Information for the User
Inaspir Accuhaler50 micrograms/inhalation, powder for inhalation
salmeterol (xinafoate)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What is Inaspir Accuhaler and what is it used for
- What you need to know before you use Inaspir Accuhaler
- How to use Inaspir Accuhaler
- Possible side effects
- Storing Inaspir Accuhaler
- Contents of the pack and further information
1. What is Inaspir Accuhaler and what is it used for
Inaspir Accuhaler belongs to a group of medicines called bronchodilators.
Inaspir Accuhaler is indicated for the relief of breathing problems in patients with asthma or chronic bronchitis (COPD).
2. What you need to know before you use Inaspir Accuhaler
Do not use Inaspir Accuhaler
- if you are allergic to salmeterol or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use Inaspir Accuhaler:
- If you have ever had to stop taking any other medication for your condition due to allergy problems or other reasons.
- If you are being treated for a thyroid disorder.
- If you are being treated for high blood pressure.
- If you are being treated for heart problems.
- If you have diabetes mellitus.
- If you have a tendency to have low potassium levels in your blood.
- If you are being treated with ketoconazole (a medicine used to treat fungal infections).
If you are using Inaspir Accuhaler for your asthma, your doctor will want to see you regularly to check your symptoms. Go to your doctor immediately if:
- Your asthma gets worse.
- You have more difficulty breathing.
- You notice more wheezing.
- You have a feeling of suffocation more often.
- You need to use your rescue medication more frequently.
If you experience any of the above situations, do not increase the number of applications of Inaspir Accuhaler (your respiratory condition may worsen and you may become seriously ill). Go to your doctor as you may need to have your medication changed for the treatment of your asthma.
Other medicines and Inaspir Accuhaler
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Important information about some of the ingredients of Inaspir Accuhaler
Use in athletes: this medicine contains salmeterol, which may produce a positive result in doping tests.
This medicine contains lactose. It may cause allergic reactions in patients with cow's milk protein allergy. If your doctor has told you that you have an intolerance to some sugars, consult with them before using this medicine.
3. How to use Inaspir Accuhaler
Follow the instructions for administration of this medicine indicated by your doctor. If in doubt, consult your doctor or pharmacist again.
Remember to use your medicine. IT IS VERY IMPORTANT TO USE IT REGULARLY EVERY DAY. This will help you stay symptom-free throughout the day and night.
Your doctor will indicate the duration of your treatment with Inaspir Accuhaler. Do not stop treatment before, even if you feel better, unless your doctor tells you to.
Inaspir Accuhaler should only be used by inhalation and should not be used in children under 4 years of age.
If you have been prescribed Inaspir Accuhaler for your asthma, you should continue to use any other medication you are taking to control your asthma. These should include an inhaled corticosteroid or tablets. Continue to take the same doses as before, unless your doctor tells you otherwise. Do this even if you feel better. Do not stop taking your inhaled corticosteroid (or tablets) when you start using Inaspir Accuhaler.
It is very important that you follow your doctor's instructions about the number of times and how often you should use Inaspir Accuhaler. The instructions for use are given below. If you have difficulties or do not understand the instructions, ask your doctor or pharmacist.
The recommended dose is:
Adults
- One inhalation (50 micrograms) twice a day. If your doctor advises, you may increase this dose up to two inhalations (100 micrograms) twice a day.
Use in children and adolescents
- Children over 4 years: one inhalation (50 micrograms) twice a day.
If you think the action of Inaspir is too strong or too weak, tell your doctor or pharmacist.
Instructions for use:
The device has two positions: closed and open.
CLOSED
When you first remove the device from the case, it will be closed.
OPEN
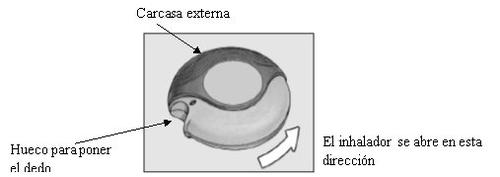
The device contains 60 individual doses of the medicine in powder form. The dose indicator shows how many doses are left.
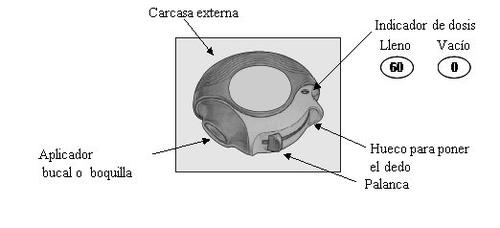
Each dose is precisely measured and hygienically protected. No maintenance or refilling is required.
The dose indicator on the top of the device shows how many doses are left. The numbers from 5 to 0 will appear in RED to warn that there are few doses left.
Handling the device is easy. When a dose is needed, follow the four simple instructions below:
- Open
- Slide
- Inhale
- Close
Device operation
When the lever on the device is slid, a small hole opens in the mouthpiece and a prepared dose is available for inhalation. When the device is closed, the lever automatically returns to its original position and is ready for the next dose you need. The external casing protects the device when not in use.
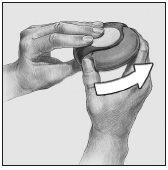
- Open
To open the device, hold the external casing with one hand and place the thumb of the other hand in the hole provided for it. Push with your thumb, moving it away from you until it stops.
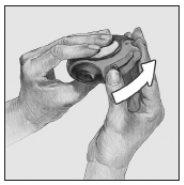
- Slide
Hold the device with the mouthpiece towards you. Slide the lever away from you until it stops, you will hear a "click" sound. The device is ready for use. Each time the lever is pulled back, a dose is available for inhalation. This is shown by the dose counter. Do not manipulate the lever, as doses will be available that will be wasted.
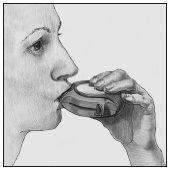
- Inhale
Before starting to inhale a dose, read this section carefully.
- Hold the device away from your mouth. Exhale (breathe out) as much as you reasonably can (do not do it inside the device).
- Place the mouthpiece in your lips. Inhale (breathe in) slowly and deeply - through the device, not through your nose.
- Remove the device from your mouth.
- Hold your breath for 10 seconds or as long as you can.
- Exhale slowly.
- You may not be able to taste or feel the powder on your tongue, even if you have used the device correctly.
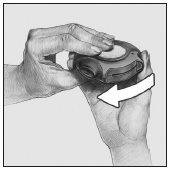
- Close
To close the device, put your thumb in the hole provided for it and slide it towards you until it stops.
When closing the device, you will hear a sharp click. The lever automatically returns to its original position and is ready to be used again.
If your doctor has prescribed two inhalations, close the device and repeat instructions 1 to 4.
REMEMBER
Keep the device dry.
Keep the device closed when not in use.
Do not exhale into the device.
Slide the lever only when you are ready to take a dose.
Do not exceed the indicated dose.
Keep out of the reach of children.
Consult your doctor or pharmacist if you have any doubts.
If you use more Inaspir Accuhaler than you should
If you have used Inaspir Accuhaler more than you should, you may notice that your heart beats faster than usual, headache, tremors, increased blood pressure, low potassium levels in the blood, feeling of agitation and/or dizziness. Consult your doctor or pharmacist immediately or the Toxicology Information Service, phone: 91 562 04 20. If the dose taken was very high, go to the doctor immediately or the Emergency Department of the nearest hospital. Bring this leaflet or the medicine with you.
If you forget to use Inaspir Accuhaler
If you forget a dose, do not worry. Inhale a dose when you remember and then continue as before.
Do not take a double dose to make up for forgotten doses.
If you have any other questions about the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some people may be allergic to medicines. If you have any of the following symptoms soon after using Inaspir Accuhaler, STOP using this medicine and tell your doctor immediately:
- Sudden onset of wheezing or chest tightness.
- Swelling of the eyelids, face or lips.
- Rash on the skin (hives) or urticaria anywhere on the body.
Some people, particularly those taking high doses of this type of medicine, may occasionally feel a little agitated, have a headache or notice that their heart beats a little faster than normal, but these effects usually disappear with continued treatment. If this feeling continues, tell your doctor but do not stop treatment unless they tell you to.
The following are side effects associated with salmeterol. Tell your doctor if you have any of the following symptoms:
Common side effects (may affect up to 1 in 10 people)
- Tremors and headache. These are characteristics of this type of medication and usually disappear over time. Tremors occur more frequently if you receive doses higher than 50 micrograms, twice a day.
- Palpitations, which usually disappear over time.
- Muscle cramps.
Uncommon side effects (may affect up to 1 in 100 people)
- Hypersensitivity reactions with skin rash.
- Nervousness.
- Tachycardia (occurs more frequently if you receive doses higher than 50 micrograms, twice a day).
Rare side effects (may affect up to 1 in 1,000 people)
- Hypokalemia (low potassium levels in the blood).
- Insomnia.
- Dizziness.
Very rare side effects (may affect up to 1 in 10,000 people)
- Hypersensitivity reactions including edema (swelling) and angioedema (skin reaction with redness, swelling, itching and difficulty breathing), bronchospasm (contraction of the bronchi causing difficulty breathing) and anaphylactic shock (severe allergic reaction).
- Hyperglycemia (high blood sugar levels). If you have diabetes, you may need to check your blood sugar levels more frequently and may need to adjust your diabetes treatment.
- Cardiac arrhythmias, including atrial fibrillation, supraventricular tachycardia and extrasystoles (heart rhythm disorders).
- Irritation of the throat or pharynx, paradoxical bronchospasm (narrowing of the bronchi with decreased air intake and difficulty breathing).
- Nausea.
- Arthralgia (joint pain).
- Unspecific chest pain.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Inaspir Accuhaler
Put the mouthpiece protector on by pushing it firmly and closing it with a snap so that the cap is in place.
Keep this medicine out of the sight and reach of children.
Do not store above 30°C.
Keep protected from heat and direct sunlight.
Keep protected from moisture.
Do not use this medicine after the expiry date that appears on the packaging after EXP. The expiry date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medicine in the SIGRE collection point at your pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and further information
Composition of Inaspir Accuhaler
- The active ingredient is 50 micrograms of salmeterol (as xinafoate) per application.
- The other ingredient is lactose monohydrate (contains cow's milk proteins).
Appearance of the product and packaging contents
Inaspir Accuhaler is a powder for inhalation. Each inhalation device contains 60 individual doses.
Marketing authorization holder and manufacturer
Marketing authorization holder:
GlaxoSmithKline, S.A.
P.T.M. C/ Severo Ochoa, 2
28760 Tres Cantos (Madrid)
Tel: +34 900 202 700
Manufacturer:
Glaxo Wellcome Productions, S.A.S.
23, Rue Lavoisier - Zone Industrielle Nº 2
Evreux La Madeleine - France
Or
Glaxo Wellcome, S.A.
Avda. de Extremadura, 3
Aranda de Duero (Burgos) - Spain
Date of last revision of this leaflet:September 2020.
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price29.58 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to INASPIR ACCUHALER 50 micrograms/inhalation, POWDER FOR INHALATIONDosage form: PULMONARY INHALATION, 0.294 mg salmeterol xinafoateActive substance: salmeterolManufacturer: Glaxosmithkline S.A.Prescription requiredDosage form: PULMONARY INHALATION, salmeterol 50 mcgActive substance: salmeterolManufacturer: Glaxosmithkline S.A.Prescription requiredDosage form: PULMONARY INHALATION, 25 µg salmeterol xinafoate/applicationActive substance: salmeterolManufacturer: Glaxosmithkline S.A.Prescription required
Online doctors for INASPIR ACCUHALER 50 micrograms/inhalation, POWDER FOR INHALATION
Discuss questions about INASPIR ACCUHALER 50 micrograms/inhalation, POWDER FOR INHALATION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















