IMPLANON NXT 68 mg IMPLANT

How to use IMPLANON NXT 68 mg IMPLANT
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is Implanon NXT and what is it used for
- What you need to know before you start using Implanon NXT
- How to use Implanon NXT
- Possible side effects
- Storage of Implanon NXT
- Container Content and Additional Information
- 1 When to Insert Implanon NXT
- 3 How to Remove Implanon NXT
- 4 How to Replace Implanon NXT
Introduction
Package Leaflet: Information for theuser
ImplanonNXT68mg implant
etonogestrel
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is not listed in this leaflet. See section 4.
Your doctor will give you the Patient Information Card that contains important information that you should know. Keep the card in a safe place and show it to your healthcare professional at any visit related to the use of the implant.
Contents of the package leaflet
- What is Implanon NXT and what is it used for
- What you need to know before you start using Implanon NXT
- How to use Implanon NXT
- Possible side effects
- Storage of Implanon NXT
- Contents of the pack and other information
Information for healthcare professionals
1. What is Implanon NXT and what is it used for
Implanon NXT is a preloaded contraceptive implant in a disposable applicator. Its efficacy and safety have been established in women between 18 and 40 years of age. The implant is a small, soft, and flexible plastic rod that measures 4 cm in length and 2 mm in diameter and contains 68 milligrams of the active substance etonogestrel. The applicator allows the healthcare professional to insert the implant just under the skin of the upper arm. Etonogestrel is a synthetic female hormone similar to progesterone. A small amount of etonogestrel is continuously released into the bloodstream. The implant is made of a copolymer of vinyl acetate-ethylene, a plastic that does not dissolve in the body. It also contains a small amount of barium sulfate, which makes it visible in X-rays.
ImplanonNXTis used to prevent pregnancy
How Implanon NXT works
The implant is inserted just under the skin. The active substance, etonogestrel, works in two ways:
- It prevents the release of an egg from the ovaries.
- It causes changes in the cervix that make it difficult for sperm to enter the uterus.
As a result, Implanon NXT protects you from pregnancy for a period of three years, but if you are overweight, your doctor may recommend replacing the implant before three years. Implanon NXT is one of the different contraceptive methods available. Another common method of birth control is the Combined Oral Contraceptive Pill. Unlike Combined Pills, Implanon NXT can be used by women who cannot or do not want to use estrogens. When using Implanon NXT, you do not need to remember to take a daily pill.
This is one of the reasons why Implanon NXT is very reliable (more than 99% effective). If in some rare cases the implant is not inserted correctly or has not been inserted at all, you may not be protected against pregnancy. While using Implanon NXT, your menstrual bleeding may change and disappear, become irregular, appear less or more often, be prolonged, or in rare cases, be intense. Your bleeding pattern during the first three months usually indicates your future bleeding pattern. Menstrual pain may improve.
You can stop using Implanon NXT at any time (see also “If you want to stop using ImplanonNXT”).
2. What you need to know before you start using Implanon NXT
Hormonal contraceptives, including ImplanonNXT, do not protect against HIV infection (AIDS) or any other sexually transmitted disease.
Do not use ImplanonNXT
Do not use Implanon NXT if you have any of the following conditions. If you have any of these, tell your doctor before Implanon NXT is inserted. Your doctor may advise you to use a non-hormonal contraceptive method.
- if you are allergic to etonogestrel or any of the other ingredients of this medicine (listed in section 6).
- if you have a blood clot. A blood clot is a clotting of blood in a blood vessel [for example in the legs (deep vein thrombosis) or in the lungs (pulmonary embolism)].
- if you have or have had jaundice (yellowing of the skin), severe liver disease (when the liver is not working properly), or a liver tumor.
- if you have had breast cancer or cancer of the genitals or there is a suspicion that you might have it.
- if you have any unexplained vaginal bleeding.
If any of these conditions appear for the first time while using ImplanonNXT, you should contact your doctor immediately.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using Implanon NXT.
If you are going to have Implanon NXT inserted and you have any of the diseases described below, you may need special medical supervision. Your doctor will explain what you need to do. If you are affected by any of these diseases, tell your doctor before Implanon NXT is inserted. Also, inform your doctor if you are diagnosed with any of these diseases during treatment with Implanon NXT or if you already had them and they worsen while you are using Implanon NXT.
- if you have had breast cancer;
- if you have or have had liver disease;
- if you have ever had a blood clot;
- if you have diabetes;
- if you are overweight;
- if you have epilepsy;
- if you have tuberculosis;
- if you have high blood pressure;
- if you have or have had chloasma (yellowish-brown patches on the skin, particularly on the face); in this case, you should avoid intense sun exposure or ultraviolet radiation.
Possibly serious situations
Cancer
The following information has been obtained from studies with women who took daily combined oral contraceptives containing two different female hormones (“The Pill”). It is not known if this data can affect women who use a different type of hormonal contraceptive, such as implants that only contain progestogen.
A slightly higher frequency of breast cancer cases has been observed in women who use combined oral contraceptives, although it is not known if this is due to treatment. For example, it could be that more tumors are found in women who use combined oral contraceptives because they go to medical check-ups more frequently. This increased frequency decreases gradually after stopping the combined oral contraceptive. It is essential to examine your breasts regularly and inform your doctor if you notice any lump in your breast. Also, inform your doctor if any of your close relatives have or have had breast cancer.
Rarely, cases of benign liver tumors, and even more rarely, malignant liver tumors have been reported in women who use The Pill. If you have severe abdominal pain, contact your doctor immediately.
Thrombosis
A blood clot in a vein (called venous thrombosis) can block the vein. It can occur in the veins of the legs, lungs (pulmonary embolism), or other organs. A blood clot in an artery (called arterial thrombosis) can block the artery. For example, a blood clot in an artery can cause a heart attack, or in the brain, it can cause a stroke.
The use of any combined hormonal contraceptive increases the risk in women of developing these clots, compared to women who do not use combined hormonal contraceptives.
The risk is not as high as the risk of developing a blood clot during pregnancy.
It is believed that the risk in users of progestogen-only contraceptives, such as Implanon NXT, is lower than in users of pills that also contain estrogens. Cases of blood clots, such as pulmonary embolism, deep vein thrombosis, heart attack, and stroke, have been reported in women who use etonogestrel implants. However, available data do not indicate an increased risk of these events in women who use the implant.
If you notice possible signs of thrombosis, you should contact your doctor immediately(see also “When to contact your doctor”).
Other situations
Changes in menstrual bleeding pattern
As with other progestogen-only contraceptives, your menstrual bleeding pattern may change during the use of Implanon NXT. You may experience changes in the frequency of bleeding (absence, decrease, more frequent or continuous bleeding), in intensity (decrease or increase), or in duration. 1 in 5 women do not have bleeding, while 1 in 5 women experience frequent and/or prolonged bleeding. Occasionally, cases of heavy bleeding have been reported. In clinical trials, changes in bleeding were the most common reason for stopping treatment (approximately 11%). Your bleeding pattern during the first three months usually indicates your future bleeding pattern.
A change in bleeding pattern does not mean that Implanon NXT is not suitable for you or that it does not protect you from pregnancy. In general, no action is necessary. You should consult your doctor if menstrual bleeding is heavy or prolonged.
Problems related to the insertion and removal of the implant
The implant may move in the arm from its initial insertion site for various reasons, such as incorrect insertion or external causes (e.g., manipulation of the implant or contact sports). In rare cases, implants have been found in the blood vessels of the arm or in the pulmonary artery (a blood vessel in the lung). In cases where the implant has moved from the initial insertion site, locating the implant may be more difficult, and removal may require a larger incision or a surgical removal procedure in the hospital. If the implant cannot be found in the arm, your healthcare professional may take an X-ray or use other imaging techniques to locate it in the chest. If the implant is found in the chest, surgery may be necessary. If the implant cannot be located and there is no evidence that it has been expelled, the contraceptive effect and the risk of progestogen-related side effects may last longer than desired.
If at any time you cannot feel the implant, you should contact your doctor as soon as possible.
Psychiatric disorders
Some women who use hormonal contraceptives like Implanon NXT have reported depression or a depressed mood. Depression can be severe and sometimes may induce suicidal thoughts. If you experience mood changes and depressive symptoms, contact your doctor for additional medical advice as soon as possible.
Ovarian cysts
With the use of all low-dose hormonal contraceptives, small fluid-filled blisters can develop in the ovaries, called ovarian cysts, which usually disappear on their own. Occasionally, they can cause mild abdominal pain and only rarely can cause more serious problems.
Broken or bent implant
The functioning of the implant should not be affected if the implant breaks or bends while it is inserted in your arm. The implant may break or bend due to external forces. A broken implant may move from its insertion site. If you have any doubts, consult your healthcare professional.
Using Implanon NXT with other medicines
Always inform your doctor about what medicines or herbal products you are using. Also, inform any doctor or dentist who prescribes you another medicine (or pharmacist) that you are using Implanon NXT. They will be able to inform you if you need to take any additional contraceptive measures (for example, using condoms) and if so, for how long, or if you need to modify the use of the other medicine.
Some medicines
- may influence Implanon NXT levels in the blood
- may make Implanon NXT less effective in preventing pregnancy
- may cause unexpected bleeding.
These include medicines used to treat:
- epilepsy (for example, primidone, phenytoin, barbiturates, carbamazepine, oxcarbazepine, topiramato, felbamato),
- tuberculosis (for example, rifampicin),
- HIV-SIDA infections (ritonavir, nelfinavir, nevirapine, efavirenz),
- Hepatitis C virus infections (for example, boceprevir, telaprevir);
- medicines for other infections (griseofulvina),
- high blood pressure in the blood vessels of the lungs (bosentan),
- depressive mood (e.g., St. John's Wort (Hypericum perforatum)).
Implanon NXT may affect the effect of other medicines, for example:
- medicines that contain ciclosporin
- the antiepileptic lamotrigina (this could lead to an increased frequency of seizures).
Consult your doctor or pharmacist before taking any medicine.
ImplanonNXTwith food and drinks
There is no indication that food and drinks affect the use of Implanon NXT.
Pregnancy and breastfeeding
Do not use Implanon NXT if you are pregnant or think you may be pregnant. If you have doubts about whether you are pregnant, you should have a pregnancy test before Implanon NXT is inserted.
Implanon NXT can be used during breastfeeding. Although a small amount of the active substance of Implanon NXT passes into breast milk, it does not affect milk production or quality, nor the growth or development of the child.
If you are breastfeeding, consult your doctor before using this medicine.
Children and adolescents
The safety and efficacy of Implanon NXT have not been established in adolescents under 18 years of age.
Driving and using machines
There are no data to suggest that the use of Implanon NXT affects the ability to drive or use machines.
When to contact your doctor
Regular check-ups Before Implanon NXT is inserted, your healthcare professional will ask you some questions about your medical history (and that of your close relatives). They will also take your blood pressure and, depending on your personal situation, may ask for other tests. During the use of Implanon NXT, your doctor may ask for another check-up (routine) some time after the implant is inserted. In general, the frequency and characteristics of follow-up checks will depend on your personal situation. Your healthcare professional should palpate the implant at each check-up visit. Contact your doctor as soon as possible in the following cases:
|
3. How to use Implanon NXT
Tell your healthcare professional if you are pregnant or think you may be pregnant before Implanon NXT is insertedNXT(for example, if you have had unprotected sex in your current menstrual cycle).
How to use
Implanon NXT should be inserted and removed only by a healthcare professional who is familiar with the procedures described in section 7 of this prospectus.
Your healthcare professional will decide with you the most suitable time for insertion. This depends on your personal situation (for example, the contraceptive method you are currently using). Unless you are switching from another hormonal contraceptive method, insertion will take place on days 1-5 of your spontaneous menstrual bleeding, to rule out pregnancy. If the implant is placed after the fifth day of menstruation, you should use an additional contraceptive method (such as a condom) for the first 7 days after insertion.
Before inserting or removing Implanon NXT, you will be given local anesthesia. Implanon NXT is inserted just under the skin, on the inner side of the upper arm of the non-dominant arm (the arm you do not use to write). The procedures for inserting and removing Implanon NXT are shown in section 6 of the prospectus.
The implant must be palpable after insertion
Once the insertion is complete, your healthcare professional will ask you to check by feeling that the implant is actually inserted in your arm (feeling the implant under the skin). A correctly inserted implant should be clearly palpable by the healthcare professional and by you, and you should be able to feel its ends between your index and thumb fingers. It should be noted that palpation does not provide total verification of the presence of the implant. If you cannot feel the implant immediately after insertion, or at any time, it may be that the implant was not inserted, it may have been inserted too deeply, or it may have moved from the place where it was inserted.
Therefore, it is essential that you occasionally palpate the implant gentlyto make sure you know where it is. If you cannot feel the implant at any time, contact your doctor as soon as possible.
If you have the slightest doubt, you should use a barrier contraceptive method (for example, a condom) until your healthcare professional and you are completely sure that the implant is inserted. Your healthcare professional may need to take an X-ray, an ultrasound, or an MRI, or ask you to have blood tests to ensure that the implant is inserted. If the implant cannot be found in the arm after a thorough search, your healthcare professional may take an X-ray or use other imaging methods to locate it in the chest. Once the healthcare professional has located the non-palpable implant, it must be removed.
ImplanonNXTmust be removed or replaced before three years after insertion.
Patient Information Card
To help you remember when and where Implanon NXT was inserted, as well as the removal deadline, your healthcare professional will give you a Patient Information Card with this information. The Patient Information Card also includes instructions for you to occasionally palpate the implant gently to make sure you know where it is. If you cannot feel the implant at any time, contact your doctor as soon as possible. Keep the card in a safe place. Show the Patient Information Card to your healthcare professional at any visit related to the use of the implant.
If you want to have a new Implanon NXT inserted at the end of the use of the one you are wearing, the new implant should be inserted immediately after the previous one has been removed. The new implant can be inserted in the same arm, often in the same place as the previous implant, as long as the site is in the correct location. Consult your healthcare professional.
If you want to stop using ImplanonNXT
You can ask your healthcare professional to remove the implant at any time you want.
If the implant cannot be located through palpation, your healthcare professional may take an X-ray, an ultrasound, or use magnetic resonance systems to locate the implant. Depending on the exact position of the implant, removal may be more complicated, possibly requiring surgery.
If you do not want to become pregnant after the removal of Implanon NXT, consult with healthcare professionals about other reliable methods of birth control.
If you stop using Implanon NXT because you want to become pregnant, it is generally recommended to wait for a menstrual period before trying to conceive. This will help you calculate the time of delivery.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
During the use of Implanon NXT, menstrual bleeding may be irregular. This bleeding may consist of only a slight spotting that does not require the use of pads. It may also consist of more intense bleeding similar to a light menstrual period that requires the use of pads. It may also happen that you do not bleed at all. Irregular bleeding is not a sign that the contraceptive protection of Implanon NXT has decreased. Normally, you will not need to take any action. However, consult your doctor if the bleeding is heavy and prolonged.
Serious side effects are explained in section 2 "Cancer" and "Thrombosis". Read this section to know the information in detail and consult your doctor immediately if you consider it necessary.
The following side effects have been reported:
Very Common (may affect more than 1 in 10 people) | Common (may affect up to 1 in 10 people) | Uncommon (may affect up to 1 in 100 people) |
Acne; headache; weight gain; breast pain and tension; irregular bleeding; vaginal infection. | Hair loss; dizziness; depressive mood; emotional instability; nervousness; decreased libido; increased appetite; stomach pain; nausea; flatulence; menstrual pain; weight loss; flu-like syndrome; pain; fatigue; hot flashes; implant site pain; implant site reaction; ovarian cyst. | Itching; genital itching; rash; increased hair growth; migraine; anxiety; difficulty sleeping; sleepiness; diarrhea; vomiting; constipation; urinary tract infection; vaginal discomfort (e.g., discharge); breast enlargement; breast secretion; back pain; fever; fluid retention; difficulty or pain when urinating; allergic reaction; throat inflammation and pain; rhinitis; joint pain; muscle pain; bone pain. |
Aside from these side effects, an increase in blood pressure has been occasionally observed. An increase in pressure in the head (benign intracranial hypertension) with signs such as persistent headache, along with a feeling of nausea, vomiting, and changes in vision, including blurred vision, has been reported.
Seborrhea (oily skin) has also been observed as a side effect. Seek medical attention immediately if you experience symptoms of a severe allergic reaction, such as (i) swelling of the face, tongue, or throat; (ii) difficulty swallowing; or (iii) hives and breathing difficulties.
During the insertion or removal of Implanon NXT, bruising (severe in some cases), pain, swelling, or itching may occur, and in rare cases, infection. A scar or abscess may form at the implant site. Due to the insertion of the implant, you may experience loss of consciousness. Swelling or a feeling of numbness (or lack of sensitivity) may occur. It is possible that the implant may be expelled or displaced if it has not been inserted correctly. In rare cases, implants have been reported to be located in a blood vessel, including a blood vessel in the lung, which may be associated with difficulty breathing and/or coughing with or without bleeding. Surgery may be necessary to remove the implant.
Cases of blood clots in a vein (called venous thrombosis) or in an artery (called arterial thrombosis) have been reported in women using etonogestrel implants. A blood clot in a vein can block the vein and can occur in the veins of the legs (deep vein thrombosis), the lungs (pulmonary embolism), or other organs. A blood clot in an artery can block the artery and can cause a heart attack or, in the brain, can cause a stroke.
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if it is a possible side effect not listed in this prospectus.
Reporting side effects:
If you experience any side effects, consult your doctor or pharmacist, even if it is a possible side effect not listed in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Implanon NXT
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the packaging (blister pack and carton).
Store in the original blister pack.
Medicines should not be disposed of via wastewater or household waste. Deposit the packaging and medicines you no longer need at the SIGRE collection point in the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
No special storage conditions are required.
6. Container Content and Additional Information
Composition of Implanon NXT
Each applicator contains an implant with
- The active ingredient is: etonogestrel (68 mg).
- The other components are: copolymer of vinyl acetate and ethylene, barium sulfate, and magnesium stearate.
Appearance of the Product and Container Content
Implanon NXT is a long-acting contraceptive for subcutaneous insertion. It consists of a radiopaque implant that only contains progestogen and is presented preloaded in a new applicator, ready to use, easy to use, and disposable.
The implant is whitish in color, measures 4 centimeters in length and 2 millimeters in diameter, and contains etonogestrel and barium sulfate. The applicator has been designed to facilitate the insertion of the implant just under the skin, in the upper-internal area of your non-dominant arm (the arm that is not used for writing). The implant must be inserted and removed by a qualified healthcare professional who is familiar with both procedures. To easily remove the implant, it is necessary for it to be inserted just under the skin. A local anesthetic should be applied before inserting or removing the implant. The risk of complications is small if the instructions provided here are followed.
Package sizes: Box with 1 blister, box with 5 blisters.
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
Organon Health, S.L.
Paseo de la Castellana, 77
28046 Madrid
Spain
Tel.: 915911279
Manufacturer:
N.V. Organon
Kloosterstraat 6,
5349 AB Oss,
Netherlands
This medicinal product is authorized in the Member States of the European Economic Areaand in the United Kingdom (Northern Ireland)under the following names:
Germany, Austria, Belgium, Slovakia, Spain, Ireland, Luxembourg, Malta, Netherlands, Poland, Portugal: Implanon NXT
Denmark, Estonia, Finland, France, Iceland, Italy, Norway, United Kingdom (Northern Ireland), Romania, Sweden: Nexplanon
Date of the last revision of thisleaflet:May 2024.
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/)
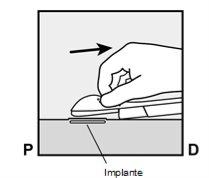
The purpose of these pictograms is only to illustrate the procedures for the insertion and removal of the implantfor the womanwho will use the implant.
Note: The exact procedures for the insertion and removal of Implanon NXTby a qualified healthcare professional are described in the Summary of Product Characteristics and in Section 7, on the other side of this leaflet.
- How to Insert Implanon NXT
- The insertion of Implanon NXT must be performed only by a qualified healthcare professional who is familiar with the technique.
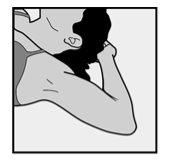
- To facilitate the insertion of the implant, lie on your back, with your arm flexed at the elbow and your hand under your head (or as close as possible).
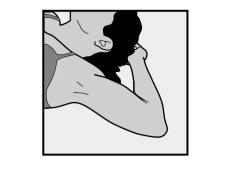
- The implant will be inserted on the inner side of the non-dominant arm (the one that is not used for writing).
- The skin will be marked at the insertion site, and the site will be disinfected and anesthetized.
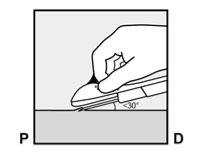
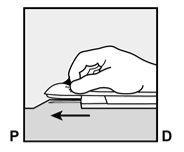
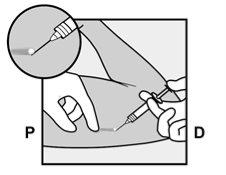
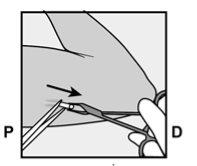
- The skin will be stretched, and the needle will be inserted directlyunder the skin. Once the tip is inside the skin, it will be inserted completely in a parallel movement to the skin.
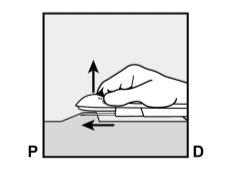
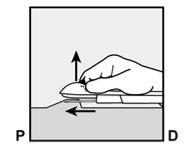
P, proximal (toward the shoulder);
D, distal (toward the elbow)
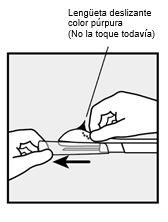
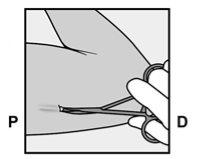
- The purple sliding tab will be unlocked to retract the needle. The implant will remain in the upper part of the arm when the needle is removed.
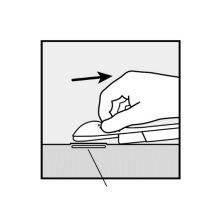
- The presence of the implant must be verified by touching the area where it has been implanted immediately after insertion (palpation). In a correctly inserted implant, the healthcare professional and you will be able to feel its ends between the index and thumb fingers. It should be noted that palpation does not guarantee 100% the presence of the implant.
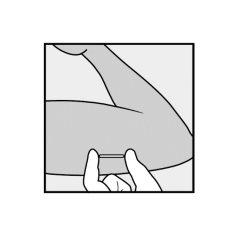
- In the event that the implant cannot be palpated or its presence is doubtful, other methods should be used to confirm the presence of the implant.
- Once the healthcare professionalhas located the implant that could not be palpated, it must be removed.
- Until the presence of the implant is verified, you may not be protected against a possible pregnancy, and therefore, a barrier contraceptive method (e.g., condom) should be used.
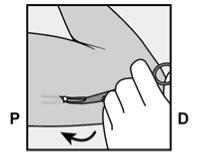
- A small adhesive bandage will be placed over the insertion site, and to minimize bruising (hematoma), a compressive bandage will be applied. You can remove the compressive bandage in 24 hours and the small inner adhesive bandage over the point where the insertion was made within 3-5 days.
- After the insertion of the implant, the healthcare professional will give you a Patient Information Card, which will indicate the insertion site, the insertion date, and the maximum date on which the implant must be removed or replaced. Keep this card in a safe place, as the information it contains may be useful for subsequent removal.
- How to Remove Implanon NXT
- The removal of the implant must be performed only by a qualified healthcare professional who is familiar with the technique.
- The implant can be removed when you request it or at most three years after it has been inserted.
- The location of the implant insertion site is indicated on the Patient Information Card.
- The healthcare professional will locate the implant. If the implant cannot be located, the healthcare professional may need to perform an X-ray, a CT scan, an ultrasound, or an MRI.
- To facilitate the removal of the implant, you should lie on your back, with your arm flexed at the elbow and your hand under your head (or as close as possible).
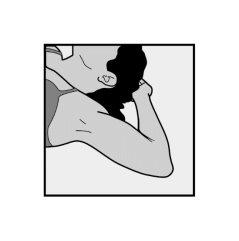

- The upper area of your arm will be disinfected and anesthetized.
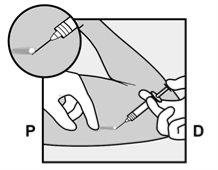
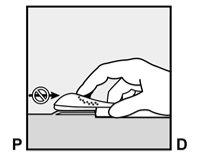
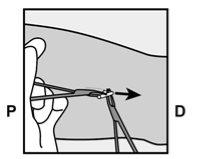
- A small incision will be made along the arm, just below the end of the implant.
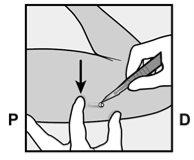
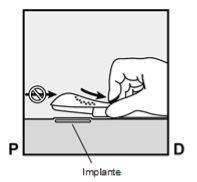
- The implant will be gently pushed toward the incision site and removed with forceps.
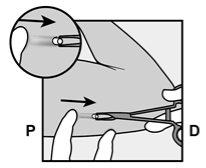
- Occasionally, the implant may be surrounded by a harder tissue layer. In this case, a small cut in the tissue will be necessary before removing the implant.
- If you want your healthcare professional to replace Implanon NXT with another implant, the new implant can be inserted in the same incision while the site is in the correct location.
- The incision will be closed with sterile skin adhesive sutures.
- A compressive bandage will be applied to minimize bruising (hematoma). You can remove the compressive bandage in 24 hours and the sterile skin adhesive sutures over the point where the insertion was made within 3-5 days.
The following information is intended only for thehealthcare professional.
- Information for the healthcare professional
7.1 When to Insert Implanon NXT
IMPORTANT: Pregnancy must be ruled out before inserting the implant.
The time of insertion depends on the woman's recent history of contraceptive use, as follows:
If the user has not been using hormonal contraception in the last month
The implant must be inserted between Day 1 (first day of menstruation) and Day 5 of the woman's menstrual cycle, even if the woman is still bleeding.
If the insertion is performed according to the indicated instructions, there is no need for a complementary contraceptive method. In case of any deviation from the appropriate time for implant insertion, it is recommended that the woman use a barrier contraceptive method for 7 days after implant insertion. If sexual intercourse has already occurred, pregnancy should be ruled out.
Switching from another hormonal contraceptive to Implanon NXT
Switching after a combined hormonal contraceptive (combined oral contraceptive, vaginal ring, or transdermal patch)
The implant should be inserted preferably on the day after the last active tablet (the last tablet with active ingredients) of the previous combined oral contraceptive or on the day of removal of the vaginal ring or transdermal patch. At the latest, the implant should be inserted the day after the rest period of any of the previously indicated contraceptive treatments or the period of administration of inactive tablets (placebo) of the previous combined oral contraceptive. Not all contraceptive methods (transdermal patch, vaginal ring) are marketed in all countries.
If the insertion is performed according to the indicated instructions, there is no need for a complementary contraceptive method. In case of any deviation from the appropriate time for implant insertion, it is recommended that the woman use a barrier contraceptive method for 7 days after implant insertion. If sexual intercourse has already occurred, pregnancy should be ruled out before insertion.
Switching after a progestogen-only contraceptive (e.g., progestogen-only pill, injectable, other implant, or intrauterine progestogen release system [IUS])
Since there are several types of progestogen-only contraceptives, the implant insertion should be performed as follows:
- Injectable contraceptives: Insert the implant on the day the next injection is due.
- Progestogen-only pill: A woman using a progestogen-only pill can switch to Implanon NXT on any day of the month. The implant must be inserted within 24 hours after taking the last tablet.
- Implant/IUS: Insert the implant on the same day the previous implant or IUS is removed.
If the insertion is performed according to the indicated instructions, there is no need for a complementary contraceptive method. In case of any deviation from the appropriate time for implant insertion, it is recommended that the woman use a barrier contraceptive method for 7 days after implant insertion. If sexual intercourse has already occurred, pregnancy should be ruled out before insertion.
After a planned or spontaneous abortion
The implant can be inserted immediately after a planned or spontaneous abortion.
- First trimester: If inserted within 5 days, there is no need for a complementary contraceptive method.
- Second trimester: If inserted within 21 days, there is no need for a complementary contraceptive method.
If the implant is inserted after the appropriate time for insertion, it is recommended that the woman use a barrier contraceptive method for 7 days after implant insertion. If sexual intercourse has already occurred, pregnancy should be ruled out before insertion.
After childbirth
The implant can be inserted immediately after childbirth, both in breastfeeding women and in those who are not, according to an individual benefit/risk assessment.
- If inserted within 21 days, there is no need for a complementary contraceptive method.
- If inserted after 21 days postpartum, it is recommended that the woman use a barrier contraceptive method for 7 days after implant insertion. If sexual intercourse has already occurred, pregnancy should be ruled out before insertion.
7.2How to Insert ImplanonNXT
The basis for successful use and subsequent removal of Implanon NXT consists of the correct and careful subcutaneous insertion of the implant in the non-dominant arm, according to the instructions indicated below. Both the healthcare professional and the woman should be able to palpate and feel the implant under the woman's skin after its placement.
The implant must be inserted subcutaneously just under the skin on the inner side of the upper non-dominant arm.
- An implant inserted at a greater depth than the subdermal layer (deep insertion) may not be palpable and may make its location and/or removal difficult (see section 4.2 How to Remove Implanon NXT and section 4.4 of the Technical Data Sheet).
- If the implant is inserted deeply, neurological or vascular damage may occur. Cases of deep or incorrect insertions have been associated with paresthesia (due to neurological damage) and displacement of the implant (due to intramuscular or fascial insertion), and in rare cases with intravascular insertion.
The insertion of Implanon NXT must be performed under aseptic conditions and only by a qualified healthcare professional who is familiar with the technique. The insertion of the implant must be performed only with the pre-loaded applicator.
Insertion Procedure
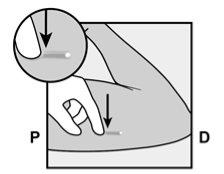
To help ensure that the implant is inserted just under the skin, the healthcare professional should position themselves in a way that allows them to verify the advancement of the needle by viewing the applicator from the side and not from above the arm. From the lateral view, the insertion site and the movement of the needle just under the skin can be clearly visualized.
For illustrative purposes, the following figures represent the inner side of the left arm.
|
Figure 1 |
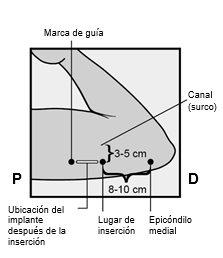
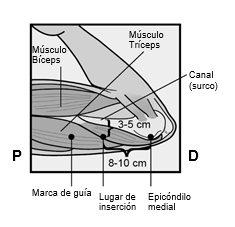
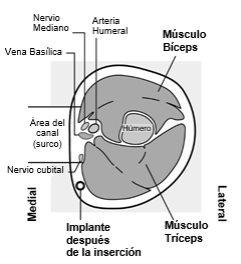
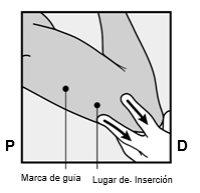
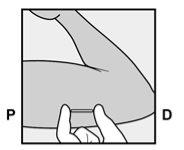
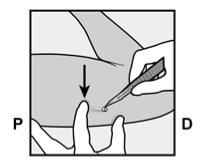
Identify the insertion site, which is located on the inner side of the upper non-dominant arm. The insertion site is situated over the triceps muscle, about 8-10 cm from the medial epicondyle of the humerus and 3-5 cm posterior (below) to the groove between the biceps and triceps muscles (Figures 2a, 2b, and 2c). This location has been selected to avoid the major blood vessels and nerves that are located inside and around the groove. If it is not possible to insert the implant at this location (for example, in women with thin arms), it should be inserted posterior to the groove and as far away from it as possible. | |
Figure 2a Figure 2b P - proximal (toward the shoulder); D - distal (toward the elbow)
Figure 2c Cross-section of the upper left arm, viewed from the elbow Medial (inner side of the arm) Lateral (outer side of the arm) | |
| |
|
Figure 3 |
To help ensure that the implant is inserted just under the skin, you should position yourself in a way that allows you to verify the advancement of the needle by viewing the applicator from the side and not from above the arm. From the lateral view, you can clearly see the insertion site and the movement of the needle just under the skin (see Figure 6). |
Figure 4
Figure 5a |
|
Figure 5b |
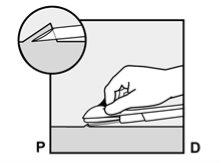
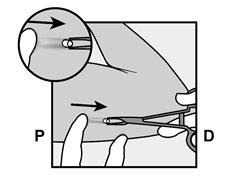
If the tip of the needle emerges from the skin before the insertion of the needle is complete, withdraw the needle backward and reposition it subcutaneously to complete the insertion procedure. |
Figure 6 |
Do not move the applicator while moving the purple sliding tab(Figure 8b). In this way, the implant is now in its final subcutaneous position, and the needle is locked inside the applicator body. You can now remove the applicator (Figure 8c). |
Figure 7
Figure 8a |
Figure 8b |
Figure 8c |
If the applicator is not maintained in the same position during this procedure or if the purple sliding tab is not moved backward completely until it stops, the implant will not be inserted correctly and may protrude from the insertion site. If the implant protrudes from the insertion site, remove the implant and, with a new applicator, perform a new procedure at the same insertion site. If the implant protrudes, do not push it back into the incision. | |
| |
|
Figure 9 |
|
|
|
If the Implant is Not Palpable After Insertion
If you cannot palpate the implant or if its presence is doubtful, it may be that the implant was not inserted or was inserted too deeply:
|
7.3 How to Remove Implanon NXT
Removal of the implant should only be performed under aseptic conditions by a qualified healthcare professional familiar with the removal technique. If you are not familiar with the removal technique, for more information, contact the Marketing Authorization Holder, Organon Health, S.L.
Before starting the removal procedure, the healthcare professional should evaluate the location of the implant. Verify the exact location of the implant in the arm by palpation.
If the implant is not palpable, consult the Patient Information Card or the patient's medical history to verify which arm the implant was inserted into. If the implant cannot be palpated, it may be inserted at a greater depth or may have been displaced. Note that the implant may be near blood vessels and nerves. Removal of non-palpable implants should only be performed by a healthcare professional with experience in removing deeply inserted implants and familiar with the location of the implant and the anatomy of the arm. For more information, contact the Marketing Authorization Holder, Organon Health, S.L.
See the section "Location and Removal of a Non-Palpable Implant" below if the implant is not palpable.
Procedure for Removing a Palpable Implant
For illustrative purposes, the following figures represent the inner side of the left arm.
|
Figure 10 |
|
Figure 11 P, proximal (toward the shoulder); D, distal (toward the elbow) |
|
Figure 12 |
|
Figure 13 |
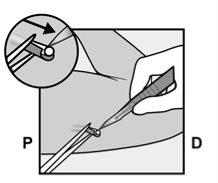
If necessary, gently remove any tissue adhering to the tip of the implant by dissecting. If the tip of the implant is not exposed after dissection, make an incision in the tissue sheath and then remove the implant with forceps (Figures 15 and 16). |
Figure 14 |
Figure 15 |
Figure 16 |
|
- If you cannot grasp the implant, stop the procedure and refer the woman to a healthcare professional with experience in complex implant removals or contact the Marketing Authorization Holder, Organon Health, S.L.
Figure 17 |
Figure 18 |
Figure 19 |
| ||
| ||
Location and Removal of a Non-Palpable Implant Occasionally, cases of implant displacement have been reported, usually minor movements in relation to the original position (see also section 4.4 of the Summary of Product Characteristics), which can result in the implant not being palpable at the site where it was inserted. A deeply inserted or displaced implant may not be palpable and therefore may require imaging techniques for localization, such as those described below. A non-palpable implant should always be located before removal. Given the radiopaque nature of the implant, suitable methods for localization include two-dimensional X-ray and computed tomography (CT) scan. High-frequency linear ultrasound (10 MHz or higher) or magnetic resonance imaging (MRI) may be used. Once the implant has been located in the arm, removal is recommended by a healthcare professional with experience in removing deeply inserted implants and familiar with the anatomy of the arm. Consider performing the removal with the help of ultrasound. If the implant cannot be found in the armafter exhaustive attempts at localization, imaging techniques in the chest may be considered, as extremely rare cases of implant displacement to the pulmonary blood vessels have been reported. If the implant is located in the chest, surgical or endovascular procedures may be necessary for removal; consult healthcare professionals familiar with thoracic anatomy. If at any time these imaging methods fail to locate the implant, its presence can be verified by determining the etonogestrel level in blood. Contact the Marketing Authorization Holder for more information. If the implant is displaced within the arm, removal may require a minor surgical procedure with a larger incision or a surgical procedure performed in the operating room. Removal of a deeply inserted implant should be performed with caution to help avoid damage to the deeper neurological or vascular structures of the arm. Non-palpable and deeply inserted implants should be removed by healthcare professionals familiar with the anatomy of the arm and with the removal of deeply inserted implants. Surgical exploration without prior knowledge of the exact location of the implant is strongly discouraged. Contact the Marketing Authorization Holder for more information. |
7.4 How to Replace Implanon NXT
After removal of the implant, it can be replaced immediately; the insertion procedure is similar to that described in section 7.2.
The new implant can be inserted in the same arm and using the same incision from which the previous implant was removed, provided the site is in the correct location, i.e., about 8-10 cm from the medial epicondyle of the humerus and 3-5 cm posterior (below) the sulcus (see section 4.2 of the Summary of Product Characteristics, How to Insert Implanon NXT). If the new implant is inserted through the same incision, anesthetize the insertion site by injecting an anesthetic (e.g., with 2 ml of lidocaine (1%)) just under the skin, starting at the incision made for removal and along the "insertion channel," and follow the steps indicated in the instructions for insertion.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to IMPLANON NXT 68 mg IMPLANTDosage form: TABLET, 75 microgramsActive substance: desogestrelManufacturer: Laboratorios Cinfa S.A.Prescription requiredDosage form: TABLET, 75 µgActive substance: desogestrelManufacturer: Gedeon Richter Plc.Prescription requiredDosage form: TABLET, 75 µgActive substance: desogestrelManufacturer: Organon Salud S.L.Prescription required
Online doctors for IMPLANON NXT 68 mg IMPLANT
Discuss questions about IMPLANON NXT 68 mg IMPLANT, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions