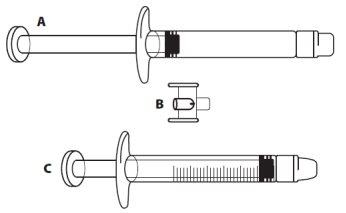
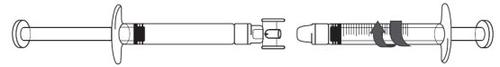
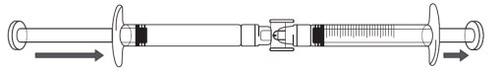
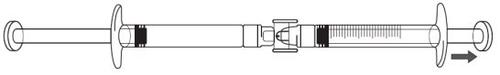
ICATIBANT EXELTIS 30 mg Injectable Solution in Pre-filled Syringe

How to use ICATIBANT EXELTIS 30 mg Injectable Solution in Pre-filled Syringe
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Icatibant Exeltis 30 mg solution for injection in pre-filled syringe EFG
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Icatibant Exeltis and what is it used for
- What you need to know before you use Icatibant Exeltis
- How to use Icatibant Exeltis
- Possible side effects
- Storing Icatibant Exeltis
- Contents of the pack and other information
1. What is Icatibant Exeltis and what is it used for
Icatibant Exeltis contains the active substance icatibant.
This medicine is used to treat the symptoms of hereditary angioedema (HAE) in adults, adolescents and children aged 2 years and above.
In HAE, the levels of a substance in the blood called bradykinin increase, leading to symptoms such as swelling, pain, nausea and diarrhoea.
This medicine blocks the action of bradykinin and therefore reduces the progression of the symptoms of an HAE attack.
2. What you need to know before you use Icatibant Exeltis
Do not use Icatibant Exeltis
- if you are allergic to icatibant or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor before you start using this medicine:
- If you have coronary heart disease (reduced blood flow to the heart).
- If you have recently had a stroke.
The side effects related to this medicine are similar to the symptoms of your own illness. Consult your doctor immediately if you notice that the symptoms of the attack worsen after you have been given this medicine.
Also:
- You or your carer must learn the technique for subcutaneous injections (under the skin) before you self-administer or your carer administers this medicine to you.
- Immediately after self-administering this medicine or after your carer has administered it to you while you are experiencing a laryngeal attack (obstruction of the upper airway), you must seek medical attention at a medical centre.
- If your symptoms do not resolve after a self-administered injection of this medicine or after an injection administered by your carer, you should consult your doctor about the administration of additional injections of this medicine. In adult patients, up to 2 additional injections can be administered within 24 hours.
Children and adolescents
This medicine is not recommended for use in children under 2 years of age or weighing less than 12 kg because it has not been studied in these patients.
Other medicines and Icatibant Exeltis
Tell your doctor if you are taking, have recently taken or might take any other medicines.
No interactions of this medicine with other medicines are known. If you are taking any medicine that is an angiotensin-converting enzyme inhibitor (e.g. captopril, enalapril, ramipril, quinapril, lisinopril) to lower your blood pressure or for any other reason, inform your doctor before using this medicine.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before using this medicine.
If you are breast-feeding, you should not breast-feed your child during the 12 hours following the last administration of this medicine.
Driving and using machines
Do not drive or use machines if you feel tired or dizzy as a result of the HAE attack or after using this medicine.
Icatibant Exeltis contains sodium
This medicine contains less than 23 mg of sodium (1 mmol) per syringe; this is essentially “sodium-free”.
3. How to use Icatibant Exeltis
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are not sure, consult your doctor again.
If you have never been administered this medicine before, the first dose must always be injected by medical or nursing staff. Your doctor will discharge you when they consider it safe for you to go home. After discussing with your doctor or nurse and after learning the technique for subcutaneous injections (under the skin), you or your carer can administer this medicine to you if you have an HAE attack. It is important to inject this medicine subcutaneously (under the skin) as soon as you notice an angioedema attack. The healthcare staff will teach you and your carer how to inject this medicine safely, following the instructions in the package leaflet.
When and how often should you use Icatibant Exeltis?
Your doctor has determined the exact dose of this medicine and will tell you how often you should use it.
Adults
- The recommended dose of this medicine is one injection (3 ml, 30 mg) administered subcutaneously (under the skin) as soon as you notice the angioedema attack (e.g. with increased skin swelling, especially on the face and neck, or increased abdominal pain).
- If you do not notice an improvement in symptoms after 6 hours, you should seek medical advice about the administration of additional injections of this medicine. In adults, up to 2 additional injections can be administered within 24 hours.
- You should not receive more than 3 injections in a 24-hour period and if you need more than 8 injections in a month, you should seek medical advice.
Children and adolescents from 2 to 17 years
- The recommended dose of this medicine is an injection of 1 ml up to a maximum of 3 ml based on body weight, administered subcutaneously (under the skin) as soon as symptoms of an angioedema attack appear (e.g. increased skin swelling, especially on the face and neck, or increased abdominal pain).
- See the instructions for use section to see the dose you should inject.
- If you are not sure about the dose you should inject, consult your doctor, pharmacist or nurse.
- If your symptoms worsen or do not improve, you should seek medical advice immediately.
How should you administer Icatibant Exeltis?
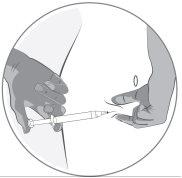
This medicine is administered by subcutaneous injection (under the skin). Each syringe should only be used once.
This medicine is injected with a short needle into the fatty tissue under the skin of the abdomen (belly). If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
The following step-by-step instructions are for:
- self-administration (adults)
- administration by a carer or healthcare professional for adults, adolescents or children over 2 years (weighing at least 12 kg).
The instructions include the following main steps:
- General information
2a) Preparation of the syringe for children and adolescents (2-17 years) weighing 65 kg or less
2b) Preparation of the syringe and needle for injection (all patients)
- Preparation of the injection site
- Injection of the solution
- Disposal of the injection materials
Step-by-step instructions for injection
- General information
- Clean the work surface (area) before you start the process.
- Wash your hands with water and soap
- Open the tray by removing the seal
- Remove the pre-filled syringe from the tray
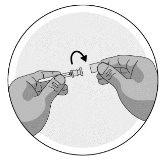
- Unscrew and remove the cap from the end of the pre-filled syringe
- Leave the pre-filled syringe once the cap has been unscrewed
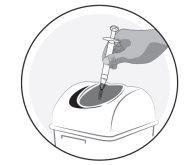
2a) Preparation of the syringe for children and adolescents (2-17 years) weighing 65 kg or less: | ||||||||||
Important information for healthcare professionals and carers: When the dose is less than 30 mg (3 ml), the following equipment is needed to extract the correct dose (see information below):
The required injection volume in ml should be prepared in an empty 3 ml graduated syringe (see table below). Table 1: Dosage schedule for children and adolescents
Patients weighing more than 65kgwill use the entire contents of the pre-filled syringe (3 ml). If you are not sure about the volume of solution to extract, consult your doctor, pharmacist or nurse
Avoid touching the ends of the connector and the tips of the syringes to prevent contamination
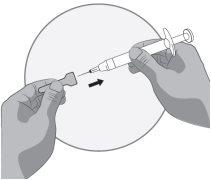
Transfer the icatibant solution to the graduated syringe:
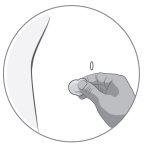
If there is airin the graduated syringe:
| ||||||||||
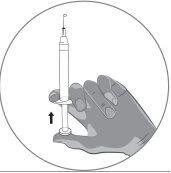
2b) Preparation of the syringe and needle for injection: All patients (adults, adolescents and children) | ||||||||||
|
|
|
|
|
|
|
|
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. Almost all patients who receive this medicine will experience a reaction at the injection site (such as skin irritation, swelling, pain, itching, redness of the skin and a burning sensation). These effects are usually mild and disappear without the need for any additional treatment.
Very common (may affect more than 1 in 10 people):
Additional reactions at the injection site (feeling of pressure, bruising, decreased sensitivity and/or numbness, increased skin rash with itching and heat).
Common (may affect up to 1 in 10 people): Nausea
Headache
Dizziness
Fever
Itching
Rash
Redness of the skin
Abnormal liver function tests
Frequency not known (cannot be estimated from the available data): Hives (urticaria)
Tell your doctor immediately if you notice that the symptoms of the attack worsen after you have received this medicine.
If you experience side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Medicines Monitoring System for Human Use: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Icatibant Exeltis
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after “EXP”. The expiry date refers to the last day of that month.
Do not store above 30°C. Do not freeze.
Do not use this medicine if you notice that the syringe or needle packaging is damaged or if you notice visible signs of deterioration; for example, if the solution is cloudy, if it contains floating particles or if the colour of the solution has changed.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Package Contents and Additional Information
Composition of Icatibant Exeltis
The active ingredient is icatibant. Each pre-filled syringe contains 30 milligrams of icatibant (as acetate). The other components are sodium chloride, glacial acetic acid, sodium hydroxide, and water for injectable preparations.
Appearance of Icatibant Exeltis and Package Contents
Icatibant Exeltis is presented as a clear and colorless injectable solution in a 3 ml pre-filled glass syringe.
The package contains a sterile subcutaneous needle.
This medication is available in a single-unit package of one pre-filled syringe with one needle or in a multi-unit package of three pre-filled syringes with three needles.
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Exeltis Healthcare S.L.
Miralcampo Avenue, 7.
Miralcampo Industrial Estate.
19200 Azuqueca de Henares. Guadalajara.
Spain.
Manufacturer
Universal Farma, S.L.
Miralcampo Industrial Estate.
El Tejido Street, 2.
19200 Azuqueca de Henares. Guadalajara.
Spain.
This medication is authorized in the member states of the European Economic Area under the following names:
Netherlands: | Icatibant Xiromed 30 mg solution for injection, in pre-filled syringe. |
Germany: | Icatibant AXiromed 30 mg injection solution in a pre-filled syringe. |
Iceland: | Icatibant Medical Valley 30 mg solution for injection, in pre-filled syringe. |
Poland: | Icatibant Medical Valley. |
Sweden: | Icatibant Medical Valley 30 mg injectable solution, in pre-filled syringe. |
Denmark: | Icatibant Medical Valley. |
Norway: | Icatibant Medical Valley. |
Spain: | Icatibanto Exeltis 30 mg injectable solution in pre-filled syringe EFG. |
Date of the last revision of this prospectus: June 2024.
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ICATIBANT EXELTIS 30 mg Injectable Solution in Pre-filled SyringeDosage form: INJECTABLE, 30 mgActive substance: icatibantManufacturer: Takeda Pharmaceuticals International Ag Ireland BranchPrescription requiredDosage form: INJECTABLE, 30 mgActive substance: icatibantManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 30 mgActive substance: icatibantManufacturer: Laboratoire AguettantPrescription required
Online doctors for ICATIBANT EXELTIS 30 mg Injectable Solution in Pre-filled Syringe
Discuss questions about ICATIBANT EXELTIS 30 mg Injectable Solution in Pre-filled Syringe, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions