
HIBERIX POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use HIBERIX POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Hiberix Powder and Solvent for Solution for Injection
Haemophilus influenzae type b Conjugate Vaccine
Read all of this leaflet carefully before your child is given this vaccine because it contains important information for your child.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This vaccine has been prescribed for your child. Do not give it to anyone else.
- If your child experiences any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Hiberix and what is it used for
- What you need to know before your child is given Hiberix
- How Hiberix is administered
- Possible side effects
- Storage of Hiberix
- Contents of the pack and further information
1. What is Hiberix and what is it used for
Hiberix is a vaccine used to protect your child against disease caused by Haemophilus influenzaetype b.
Haemophilus influenzaetype b (Hib) can cause meningitis. This can lead to serious problems such as mental retardation, cerebral palsy, deafness, epilepsy, or partial blindness. It can also cause throat inflammation that can lead to death by suffocation. Less frequently, the bacteria can also infect the blood, heart, lungs, bones, joints, and tissues of the eyes and mouth.
Hiberix is indicated for primary vaccination of children from 2 months of age, including those in the following groups:
- Children with asplenia, sickle cell anemia, or immunodeficiency.
- Children infected with HIV, asymptomatic or symptomatic.
- In situations determined by the relevant Health Authorities.
Hiberix helps your child's body create its own protection (antibodies). This will protect them against the disease.
As with all vaccines, Hiberix may not completely protect all vaccinated children.
Hiberix only protects against infections caused by Haemophilus influenzaetype b, for which the vaccine was developed.
Children with a weakened immune system (due to HIV infection, for example) may not be fully protected by Hiberix.
The vaccine cannot cause the disease it protects your child against.
2. What you need to know before your child is given Hiberix
Hiberix should not be administered
- If your child is allergic (hypersensitive) to the active substances or any of the other components of the vaccine (listed in section 6). At the end of the leaflet, there is a list of active substances and other components of Hiberix. Signs of an allergic reaction may include skin rash with itching, difficulty breathing, and swelling of the face or tongue.
Warnings and precautions
Consult your doctor or pharmacist before your child is given Hiberix if:
- your child has a severe infection with high fever. In these cases, vaccination will be postponed until recovery. A minor infection, such as a cold, should not be a problem, but consult your doctor first.
- your child has difficulty breathing, inform your doctor. This may be more frequent during the first three days after vaccination, if your child is premature (born at 28 weeks of gestation or earlier).
A faint may occur before or after any injection, so you should inform your doctor or nurse if your child has fainted after previous injections.
Use of Hiberix with other medicines
- Tell your doctor or pharmacist that your child is using, has recently used, or may need to use any other medicine, including those obtained without a prescription or if they have recently been given another vaccine.
- Inform your doctor or pharmacist especially if your child is using any medicine or has an infection that affects the immune system (the body's natural defense system), as your child may not be fully protected by Hiberix.
- Hiberix can be administered at the same time as other childhood vaccines. Different injection sites will be used for each vaccine.
Hiberix should not be mixed in the same syringe with other vaccines except with Tritanrix HepB.
Hiberix contains sodium
This medicine contains less than 23 mg (1 mmol) of sodium per 0.5 ml; it is essentially "sodium-free".
3. How Hiberix is administered
Follow exactly the administration instructions for this medicine indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
- Your doctor or nurse will administer the recommended dose for your child. This will depend on official recommendations.
- Normally, the primary vaccination schedule consists of administering 3 doses of Hiberix, separated by an interval of 1-2 months, in the first 6-7 months of life, and can start from 2 months of age.
- A booster dose may be necessary in the second year of life, for example, in children who have not completed primary vaccination. If necessary, your doctor or nurse will inform you.
- Hiberix is administered into the muscle.
- The vaccine should never be administered into a vein.
- You will be informed when your child should receive the next dose.
If more Hiberix is administered than should be
No cases of overdose have been reported. Since the package contains only one dose, overdose is unlikely.
If your child misses a dose of Hiberix
- If your child misses a scheduled dose, it is essential to request another appointment. If the administration of a dose is not carried out according to the planned schedule, the administration of this dose can be delayed, provided that the 3 doses are administered during the first year of life, maintaining an interval of 1-2 months between doses.
- If the complete cycle of vaccination of the three injections is not completed, your child may not obtain the best immune response or protection against the disease.
4. Possible side effects
Like all medicines, Hiberix can cause side effects, although not everyone gets them.
The side effects that occurred during clinical trials were:
Allergic reactions
As with all injectable vaccines, your child may experience an allergic reaction, although these are very rare (less than 1 in every 10,000 doses of vaccine).
Signs of an allergic reaction may be:
- skin rashes that can cause itching or blisters
- swelling of the eyes and face
- difficulty breathing or swallowing
- sudden drop in blood pressure
- loss of consciousness
These symptoms usually appear immediately after the injection. Take your child to the doctor immediately if these symptoms start after leaving the clinic.
Seek medical attention immediately if your child has any of the following serious side effects
Very common(may occur in more than 1 in 10 doses of the vaccine):
- irritability
- drowsiness
- fever
- swelling, pain, and redness at the injection site
- loss of appetite
- crying
- restlessness
- diarrhea
Common(may occur in up to 1 in 10 doses of the vaccine):
- vomiting
Rare(may occur in up to 1 in 1,000 doses of the vaccine):
- seizures (including febrile seizures)
Additionally, other effects not observed during clinical trials but reported after marketing of Hiberix are:
Very rare(may occur in less than 1 in 10,000 doses of vaccine):
- fainting due to injection
- collapse (sudden loss of muscle tone), periods of unconsciousness, or loss of consciousness and pale or blue-tinged skin
- temporary interruption of breathing
- urticaria, localized skin rash in one or more areas, or throughout the body
- swelling of the limb where the vaccine was injected
- hard lump at the injection site
Reporting of side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the Spanish Medicines and Healthcare Products Agency (AEMPS) website:
www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Hiberix
- Keep this medicine out of the sight and reach of children.
- Store in a refrigerator (between 2°C and 8°C).
- Do not freeze.
- Store in the original packaging to protect it from light.
- Do not use Hiberix after the expiration date stated on the package. The expiration date is the last day of the month indicated.
- Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medicines in the pharmacy's SIGRE collection point. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Contents of the pack and further information
Composition of Hiberix
- The active substances are:
Haemophilus influenzaetype b polysaccharide 10 micrograms conjugated with tetanus toxoid as a carrier protein approx. 25 micrograms
- The other components are:
Powder: lactose
Solvent: sodium chloride and water for injections
Appearance of the product and contents of the pack
Hiberix is presented as a powder in a glass vial and a solvent in a pre-filled syringe.
The powder is white, and the solvent is clear and colorless.
Marketing authorization holder
GlaxoSmithKline, S.A.
PTM - C/ Severo Ochoa, 2
28760 Tres Cantos
Madrid
Phone: 900 202 700
e-mail: [email protected]
Manufacturer
GlaxoSmithKline Biologicals S.A.
Rue de L’Institut 89; 1330 Rixensart
Belgium
or
SMITHKLINE BEECHAM, S.A.
Ctra. de Ajalvir Km. 2.5 (Alcalá de Henares (Madrid)) – 28806
Spain
Date of the last revision of this leaflet:12/2019
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) http://www.aemps.gob.es/.
----------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Before reconstitution or administration, the solvent and the reconstituted vaccine should be visually inspected for any foreign particles and/or changes in physical appearance. If any are observed, do not use the solvent or the reconstituted vaccine.
Instructions for reconstitution of the vaccine with the solvent in a pre-filled syringe
Hiberix should be reconstituted by adding the entire contents of the pre-filled syringe to the vial containing the powder.
To learn how to insert the needle into the syringe, read the instructions provided with images 1 and 2 carefully. However, the syringe provided with Hiberix may be slightly different (without a screw thread) than the syringe in the image. In this case, the needle should be inserted without screwing.
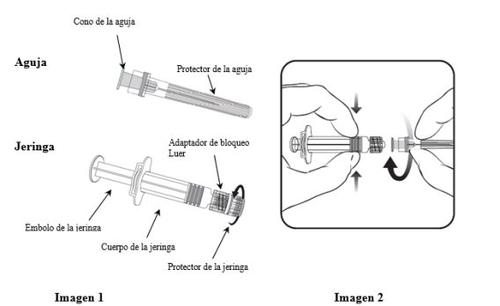
Always hold the syringe by the body, not by the plunger or the Luer adapter, and keep the needle in the axis of the syringe (as shown in image 2). Otherwise, the Luer adapter could become deformed and cause leaks.
If the Luer adapter comes off during syringe assembly, use a new dose of the vaccine (new syringe and vial).
- Unscrew the syringe protector by turning it counterclockwise (as shown in image 1).
Whether the Luer adapter turns or not, please follow the next steps:
- Gently insert the needle into the syringe by fitting the cone of the needle into the Luer adapter and turning a quarter turn clockwise until it clicks (as shown in image 2).
- Remove the needle protector (it may be difficult).
- Add the solvent to the powder. The mixture should be shaken well until the powder is completely dissolved.
The reconstituted vaccine is a clear to opalescent and colorless solution.
After reconstitution, the vaccine should be administered promptly. If not used within 8 hours after reconstitution, it should be discarded.
- A new needle should be used to administer the vaccine. Unscrew the needle from the syringe and insert the injection needle by repeating step 2.
Hiberix can be mixed in the same syringe with the monodose vaccine Tritanrix HepB. It should be checked that the vaccine to be mixed with Hiberix is in a monodose package. From the Hiberix packaging, discard the container with the solvent. In this case, the solvent included in the Hiberix packaging will be replaced by the liquid vaccine of Tritanrix HepB. The combined vaccine should be reconstituted by adding the complete contents of the Tritanrix HepB container to the vial containing the white Hib powder. This extemporaneously combined vaccine should be handled in the same way as the reconstituted monocomponent Hiberix vaccine.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to HIBERIX POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 0.5 ml single doseActive substance: meningococcus B, multicomponent vaccineManufacturer: Glaxosmithkline Vaccines S.R.L.Prescription requiredDosage form: INJECTABLE, 0.5 ml single doseActive substance: meningococcus B, multicomponent vaccineManufacturer: Glaxosmithkline Vaccines S.R.L.Prescription requiredDosage form: INJECTABLE, -Active substance: pertussis, purified antigen, combinations with toxoidsManufacturer: Glaxosmithkline S.A.Prescription required
Online doctors for HIBERIX POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about HIBERIX POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















