
HEMOSOL B0 SOLUTION FOR HEMOFILTRATION AND HEMODIALYSIS


How to use HEMOSOL B0 SOLUTION FOR HEMOFILTRATION AND HEMODIALYSIS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Hemosol B0 Solution for Haemodialysis and Haemofiltration
Sodium chloride/calcium chloride dihydrate/magnesium chloride hexahydrate/lactic acid/sodium bicarbonate.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
In this leaflet:
- What is Hemosol B0 and what is it used for
- What you need to know before you start using Hemosol B0
- How to use Hemosol B0
- Possible side effects
- Storing Hemosol B0
- Contents of the pack and other information
1. What is Hemosol B0 and what is it used for
Hemosol B0 is used in hospitals in intensive care treatments to correct the chemical imbalance of the blood caused by kidney failure. The goal of these treatments is to remove waste products from the blood that accumulate when the kidneys do not function properly.
Hemosol B0 is used in these types of treatment for adults and children of all ages:
- haemofiltration,
- haemodiafiltration, and
- haemodialysis.
2. Before you use Hemosol B0
Do not use Hemosol B0:
If you are allergic to one of the active substances or to any of the other ingredients (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using Hemosol B0.
Hemosol B0 is a hospital product that should only be administered by medical professionals. They will ensure that the medicine is used safely.
Your blood will be checked before and during treatment. For example, the acid-base balance and the concentration of salts in the blood (electrolytes) will be monitored, including all fluid inputs (intravenous perfusion) and outputs (diuresis), even those not directly related to the treatment.
Since Hemosol B0 does not contain potassium, special attention should be paid to potassium levels in the blood. If levels are low, it may be necessary to administer a potassium supplement.
Children
There are no specific warnings or precautions for children derived from the use of this medicine.
Using Hemosol B0 with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
You should do this because the concentration levels in the blood of some medicines may be reduced during treatment with Hemosol B0. Your doctor will decide if any of the medicines you are taking need to be changed.
In particular, talk to your doctor if you are taking any of the following medicines:
- Digitalis medicines (for the treatment of certain heart failures) since the risk of cardiac arrhythmias induced by these medicines increases when potassium concentration levels in the blood are low (hypokalaemia).
- Vitamin D and medicines that contain calcium since they may increase the risk of high calcium concentration in the blood (hypercalcaemia).
- Any sodium bicarbonate solution supplement (or other buffered source) since it may increase the risk of basic compounds in the blood (metabolic alkalosis).
When citrate is used as an anticoagulant, it may reduce calcium levels in the plasma.
Pregnancy, breast-feeding, and fertility
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine. No effects on fertility, pregnancy, or the unborn/newborn baby are anticipated. Your doctor will decide if you should be given Hemosol B0 if you are pregnant or breast-feeding.
Driving and using machines
Hemosol B0 does not affect the ability to drive or use machines.
3. How to use Hemosol B0
Hemosol B0 is a product that should only be administered by medical professionals.
The volume of Hemosol B0 and, therefore, the dose used will depend on your conditions. The dose volume will be determined by the doctor responsible for the treatment.
Hemosol B0 can be administered directly into the bloodstream (intravenously) or through haemodialysis, a technique in which the solution flows on one side of the dialysis membrane while the blood flows on the other.
If you use more Hemosol B0 than you should
Hemosol B0 is a hospital product that should only be administered by medical professionals. Additionally, thorough control of fluid balance, electrolyte balance, and acid-base balance is carried out.
Therefore, it is unlikely that you will use more Hemosol B0 than you should.
In the unlikely event that you are given an overdose, your doctor will take the necessary corrective measures and adjust the dose.
Overdose may result in:
- fluid overload in the blood,
- elevation of bicarbonate levels in the blood (metabolic alkalosis),
- and/or reduction of salt levels in the blood (hypophosphataemia, hypokalaemia).
To consult the instructions for use, see the section "This information is intended only for healthcare professionals".
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been reported:
Frequency not known (cannot be estimated from the available data)
- Variations in salt levels in the blood (electrolyte imbalances, such as hypophosphataemia, hypokalaemia).
- Increased bicarbonate concentration in the plasma (metabolic alkalosis) or reduced bicarbonate concentration in the plasma (metabolic acidosis).
- Abnormally high or low water volume in the body (hyper- or hypovolaemia).
- Nausea.
- Vomiting.
- Muscle cramps,
- Low blood pressure (hypotension).
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Monitoring System for Human Use: www.notificaRAM.es.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Hemosol B0
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the back of the bag and on the label after CAD. The expiry date is the last day of the month shown.
Do not store below 4°C.
The physical and chemical stability of the reconstituted solution has been demonstrated for 24 hours when stored at 22°C. From a microbiological point of view, the reconstituted solution should be used immediately. The use of the solution stored under other conditions and for other periods is the responsibility of the user and should not exceed 24 hours, including the duration of treatment.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Additional Information
HEMOSOL B0 – PVC WITH LUER CONNECTOR AND BREAKABLE VÁSTAGO
Composition of Hemosol B0
The active ingredients before and after reconstitution are:
Active ingredients before reconstitution:
1000 ml of solution from the small compartment (A)contain:
Calcium chloride, 2H2O 5.145 g
Magnesium chloride, 6 H2O 2.033 g
Lactic acid 5.4 g
1000 ml of solution from the large compartment (B)contain:
Sodium bicarbonate 3.09 g
Sodium chloride 6.45 g
Active ingredients after reconstitution:
The solutions from compartments A (250 ml) and B (4750 ml) are mixed to produce a reconstituted solution (5000 ml) with the following composition:
mmol/l
Calcium, Ca2+ 1.75
Magnesium, Mg2+ 0.5
Sodium, Na+ 140
Chloride Cl- 109.5
Lactate 3
Bicarbonate, HCO3- 32
Theoretical osmolality: 287 mOsm/l
Other components are:carbon dioxide (E-290) and water for injectable preparations.
Appearance of the product and packaging content
Hemosol B0 is presented in a two-compartment bag. The bag is covered by a transparent wrapper.
The final reconstituted solution is obtained after breaking the breakable vástago and mixing both solutions.
The reconstituted solution is transparent and colorless. Each bag (A+B) contains 5000 ml of solution for hemofiltration, hemodiafiltration, and/or hemodialysis.
Each box contains two bags and a prospectus.
Marketing authorization holder:
Vantive Belgium SRL
Boulevard d´Angleterre 2
1420 Braine-l´Alleud
Belgium
Manufacturer:
Bieffe Medital S.P.A.
Via Stelvio 94,
23035 Sondalo (SO),
Italy
or
Vantive Manufacturing Limited
Moneen Road,
Castlebar, Co. Mayo F23 XR63
Ireland
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Vantive Health, S.L.
Polígono industrial sector 14
C/ Pouet de Camilo nº2
46394 Ribarroja del Turia
Valencia
Spain
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) with the following names:
Germany, Austria, Belgium, Bulgaria, Croatia, Cyprus, Denmark, Slovakia, Slovenia, Spain, Estonia, Finland, France, Greece, Iceland, Ireland, Latvia, Lithuania, Luxembourg, Malta, Norway, Netherlands, Poland, Portugal, United Kingdom (Northern Ireland), Czech Republic, and Sweden: Hemosol B0.
Date of the last revision of this prospectus: 11/2018
__________________________________________________________________________
This information is intended only for healthcare professionals
Hemosol B0 solution for hemodialysis and hemofiltration
Precautions
Follow the instructions for use and handling of Hemosol B0 accurately.
The solutions from the two compartments mustbe mixed before use.The use of a contaminated hemofiltration solution can cause sepsis, shock, and fatal disorders.
To increase patient comfort, Hemosol B0 can be heated to 37°C. Preheating before using the solution should be done before reconstitution using only dry heat. The solutions should not be heated in water or in a microwave oven. Before administration and whenever the solution and container allow, it should be visually checked that the solution does not contain particles or has lost its original color. Do not administer the solution unless it is transparent and the seal is intact.
The addition of sodium bicarbonate for substitution may increase the risk of metabolic alkalosis.
Before and during treatment, the electrolyte and acid-base balance should be carefully monitored. Since Hemosol B0 does not contain potassium, the potassium concentration in the serum should be monitored before and after hemofiltration and/or hemodialysis. Potassium addition may be necessary.
Up to 1.2 mmol/l of phosphate can be added to the solution. If potassium phosphate is added, the total potassium concentration should not exceed 4 mEq/l (4 mmol/l).
The volume and rate at which Hemosol B0 is used will depend on the electrolyte concentration in the blood, acid-base balance, and the patient's overall clinical condition. The administration regimen (dose, perfusion rate, and cumulative volume) of Hemosol B0 should be determined by a doctor. Continuous hemofiltration application will eliminate excess fluids and electrolytes.
In case of fluid imbalance, the clinical situation should be closely monitored and fluid balance corrected as necessary.
Overdose will result in fluid overload in patients with renal failure, which could have serious consequences such as congestive heart failure or alterations in acid-base or electrolyte balance.
The solution does not contain glucose, so its administration may cause hypoglycemia. Blood glucose levels should be regularly monitored.
Hemosol B0 contains bicarbonate and lactate (a precursor of bicarbonate), which can affect the patient's acid-base balance. If metabolic alkalosis appears or worsens during treatment with the solution, it may be necessary to reduce the perfusion rate or suspend administration.
Posology
The commonly used flow rates for the substitution solution in hemofiltration and hemodiafiltration are:
Adults: 500-3000 ml/hour
The commonly used flow rates for the dialysis solution (dialysate) in continuous hemodialysis are:
Adults: 500-2500 ml/hour
The commonly used flow rates in adults are between 2000 and 2500 ml/h approximately, which corresponds to a daily fluid volume of approximately 48 to 60 L.
Pediatric population
The flow rate range for the substitution solution in hemofiltration and hemodiafiltration and for the dialysis solution (dialysate) in continuous hemodialysis is:
Children (from neonates to adolescents up to 18 years): 1000 to 2000 ml/h/1.73 m2.
Flow rates of up to 4000 ml/h/1.73 m2 may be necessary, especially in younger children (≤ 10 kg). In general, the absolute flow rate (in ml/h) in the pediatric population should not exceed the maximum flow rate for adults.
Instructions for use and handling
The electrolyte solution (compartment A small) is added to the buffer solution (compartment B large) after breaking the breakable vástago immediately before use to obtain the reconstituted solution.
Use only with suitable extracorporeal renal replacement therapy equipment.
Aseptic technique should be used throughout the handling and administration process to the patient.
Use the solution if the wrapper is not damaged, all seals are intact, the breakable vástago is not broken, and the solution is transparent. Squeeze the bag to ensure there are no leaks. If any leak is found, discard the solution immediately as its sterility cannot be guaranteed.
Compartment B large has an injection port for adding other necessary medications once the solution is reconstituted.
It is the doctor's responsibility to judge the compatibility of a medication added to the Hemosol B0 solution. For this, possible changes in color and/or precipitation, insoluble complexes, or crystals should be checked. Before adding a medication, verify if it is soluble and stable in water within the pH limits of Hemosol B0 (the pH range of the reconstituted solution is 7.0 to 8.5). Additives may not be compatible. The instructions for use of the medication to be added should be consulted.
Remove any liquid from the injection port, hold the bag in a vertical position downwards, add the medication through the injection port, and mix completely. The solution should be administered immediately. Introduction and mixing of additives should always be done before connecting the solution bag to the extracorporeal circuit.
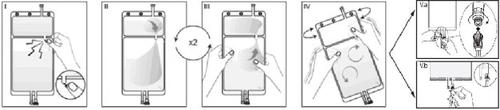
IRemove the wrapper from the bag immediately before use and discard other packaging materials. Open the seal by breaking the vástago between the two compartments of the bag. The vástago will remain inside the bag (see figure I, below).
IIEnsure that all liquid from compartment A small passes into compartment B large (see figure II, below).
IIIClarify twicecompartment A small by taking the mixed solution back to this compartment and again to compartment B large (see figure III, below).
IVOnce compartment A small is empty, shake compartment B large to mix its contents completely. The solution is now ready for use, and the bag can be hung on the equipment (see figure IV, below).
VThe dialysis or substitution line can be connected to either of the two access ports.
V.aIf the luer connector is used, remove the plug by a twisting and pulling movement and connect the luer male connector of the dialysis or substitution line to the luer female receptor of the bag by a pushing and twisting movement. Ensure the connection is secure and tight. The connection will open. Check that the liquid circulates freely (see figure V.a, below).
If the dialysis or substitution line is disconnected from the luer connector, the connector will close, and the solution flow will stop. The luer port is a needle-free port that can be cleaned.
V.bIf the injection port is used, first remove the plug by lifting it. The injection port is a port that can be disinfected with a swab. Then, insert the spike through the rubber wall. Check that the liquid circulates freely (see figure V.b, below)
The solution should be used immediately after removing the wrapper. If not used immediately, the reconstituted solution should be used within 24 hours after adding the electrolyte solution to the buffer solution, including the duration of treatment.
The reconstituted solution is for single use. Discard any remaining solution immediately after use.
Disposal of unused medication and all materials that have come into contact with it should be done according to local regulations.
----------------------------------------------------------------------------------------------------------------------
HEMOSOL B0 – PVC WITH LUER CONNECTOR AND BREAKABLE VÁSTAGO
Composition of Hemosol B0
The active ingredients before and after reconstitution are:
Active ingredients before reconstitution:
1000 ml of solution from the small compartment (A)contain:
Calcium chloride, 2H2O 5.145 g
Magnesium chloride, 6 H2O 2.033 g
Lactic acid 5.4 g
1000 ml of solution from the large compartment (B)contain:
Sodium bicarbonate 3.09 g
Sodium chloride 6.45 g
Active ingredients after reconstitution:
The solutions from compartments A (250 ml) and B (4750 ml) are mixed to produce a reconstituted solution (5000 ml) with the following composition:
mmol/l
Calcium, Ca2+ 1.75
Magnesium, Mg2+ 0.5
Sodium, Na+ 140
Chloride Cl- 109.5
Lactate 3
Bicarbonate, HCO3- 32
Theoretical osmolality: 287 mOsm/l
Other components are:carbon dioxide (E-290) and water for injectable preparations.
Appearance of the product and packaging content
Hemosol B0 is presented in a two-compartment bag. The bag is covered by a transparent wrapper.
The final reconstituted solution is obtained after breaking the breakable vástago and mixing both solutions.
The reconstituted solution is transparent and colorless. Each bag (A+B) contains 5000 ml of solution for hemofiltration, hemodiafiltration, and/or hemodialysis.
Each box contains two bags and a prospectus.
Marketing authorization holder:
Vantive Belgium SRL
Boulevard d´Angleterre 2
1420 Braine-l´Alleud
Belgium
Manufacturer:
Bieffe Medital S.P.A.
Via Stelvio 94,
23035 Sondalo (SO),
Italy
or
Vantive Manufacturing Limited
Moneen Road,
Castlebar, Co. Mayo F23 XR63
Ireland
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Vantive Health, S.L.
Polígono industrial sector 14
C/ Pouet de Camilo nº2
46394 Ribarroja del Turia
Valencia
Spain
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) with the following names:
Germany, Austria, Belgium, Bulgaria, Croatia, Cyprus, Denmark, Slovakia, Slovenia, Spain, Estonia, Finland, France, Greece, Iceland, Ireland, Latvia, Lithuania, Luxembourg, Malta, Norway, Netherlands, Poland, Portugal, United Kingdom (Northern Ireland), Czech Republic, and Sweden: Hemosol B0.
Date of the last revision of this prospectus: 11/2018
__________________________________________________________________________
This information is intended only for healthcare professionals
Hemosol B0 solution for hemodialysis and hemofiltration
Precautions
Follow the instructions for use and handling of Hemosol B0 accurately.
The solutions from the two compartments mustbe mixed before use.The use of a contaminated hemofiltration solution can cause sepsis, shock, and fatal disorders.
To increase patient comfort, Hemosol B0 can be heated to 37°C. Preheating before using the solution should be done before reconstitution using only dry heat. The solutions should not be heated in water or in a microwave oven. Before administration and whenever the solution and container allow, it should be visually checked that the solution does not contain particles or has lost its original color. Do not administer the solution unless it is transparent and the seal is intact.
The addition of sodium bicarbonate for substitution may increase the risk of metabolic alkalosis.
Before and during treatment, the electrolyte and acid-base balance should be carefully monitored. Since Hemosol B0 does not contain potassium, the potassium concentration in the serum should be monitored before and after hemofiltration and/or hemodialysis. Potassium addition may be necessary.
Up to 1.2 mmol/l of phosphate can be added to the solution. If potassium phosphate is added, the total potassium concentration should not exceed 4 mEq/l (4 mmol/l).
The volume and rate at which Hemosol B0 is used will depend on the electrolyte concentration in the blood, acid-base balance, and the patient's overall clinical condition. The administration regimen (dose, perfusion rate, and cumulative volume) of Hemosol B0 should be determined by a doctor. Continuous hemofiltration application will eliminate excess fluids and electrolytes.
In case of fluid imbalance, the clinical situation should be closely monitored and fluid balance corrected as necessary.
Overdose will result in fluid overload in patients with renal failure, which could have serious consequences such as congestive heart failure or alterations in acid-base or electrolyte balance.
The solution does not contain glucose, so its administration may cause hypoglycemia. Blood glucose levels should be regularly monitored.
Hemosol B0 contains bicarbonate and lactate (a precursor of bicarbonate), which can affect the patient's acid-base balance. If metabolic alkalosis appears or worsens during treatment with the solution, it may be necessary to reduce the perfusion rate or suspend administration.
Posology
The commonly used flow rates for the substitution solution in hemofiltration and hemodiafiltration are:
Adults: 500-3000 ml/hour
The commonly used flow rates for the dialysis solution (dialysate) in continuous hemodialysis are:
Adults: 500-2500 ml/hour
The commonly used flow rates in adults are between 2000 and 2500 ml/h approximately, which corresponds to a daily fluid volume of approximately 48 to 60 L.
Pediatric population
The flow rate range for the substitution solution in hemofiltration and hemodiafiltration and for the dialysis solution (dialysate) in continuous hemodialysis is:
Children (from neonates to adolescents up to 18 years): 1000 to 2000 ml/h/1.73 m2.
Flow rates of up to 4000 ml/h/1.73 m2 may be necessary, especially in younger children (≤ 10 kg). In general, the absolute flow rate (in ml/h) in the pediatric population should not exceed the maximum flow rate for adults.
Instructions for use and handling
The electrolyte solution (compartment A small) is added to the buffer solution (compartment B large) after breaking the breakable vástago immediately before use to obtain the reconstituted solution.
Use only with suitable extracorporeal renal replacement therapy equipment.
Aseptic technique should be used throughout the handling and administration process to the patient.
Use the solution if the wrapper is not damaged, all seals are intact, the breakable vástago is not broken, and the solution is transparent. Squeeze the bag to ensure there are no leaks. If any leak is found, discard the solution immediately as its sterility cannot be guaranteed.
Compartment B large has an injection port for adding other necessary medications once the solution is reconstituted.
It is the doctor's responsibility to judge the compatibility of a medication added to the Hemosol B0 solution. For this, possible changes in color and/or precipitation, insoluble complexes, or crystals should be checked. Before adding a medication, verify if it is soluble and stable in water within the pH limits of Hemosol B0 (the pH range of the reconstituted solution is 7.0 to 8.5). Additives may not be compatible. The instructions for use of the medication to be added should be consulted.
Remove any liquid from the injection port, hold the bag in a vertical position downwards, add the medication through the injection port, and mix completely. The solution should be administered immediately. Introduction and mixing of additives should always be done before connecting the solution bag to the extracorporeal circuit.
IRemove the wrapper from the bag immediately before use and discard other packaging materials. Open the seal by breaking the vástago between the two compartments of the bag. The vástago will remain inside the bag (see figure I, below).
IIEnsure that all liquid from compartment A small passes into compartment B large (see figure II, below).
IIIClarify twicecompartment A small by taking the mixed solution back to this compartment and again to compartment B large (see figure III, below).
IVOnce compartment A small is empty, shake compartment B large to mix its contents completely. The solution is now ready for use, and the bag can be hung on the equipment (see figure IV, below).
VThe dialysis or substitution line can be connected to either of the two access ports.
V.aIf the luer connector is used, remove the plug by a twisting and pulling movement and connect the luer male connector of the dialysis or substitution line to the luer female receptor of the bag by a pushing and twisting movement. Ensure the connection is secure and tight. The connection will open. Check that the liquid circulates freely (see figure V.a, below).
If the dialysis or substitution line is disconnected from the luer connector, the connector will close, and the solution flow will stop. The luer port is a needle-free port that can be cleaned.
V.bIf the injection port is used, first remove the plug by lifting it. The injection port is a port that can be disinfected with a swab. Then, insert the spike through the rubber wall. Check that the liquid circulates freely (see figure V.b, below)
The solution should be used immediately after removing the wrapper. If not used immediately, the reconstituted solution should be used within 24 hours after adding the electrolyte solution to the buffer solution, including the duration of treatment.
The reconstituted solution is for single use. Discard any remaining solution immediately after use.
Disposal of unused medication and all materials that have come into contact with it should be done according to local regulations.

Sterility.
The large compartment B has an injection port to add other medications that may be necessary once the solution is reconstituted.
It is the responsibility of the physician to judge the compatibility of an added medication to the Hemosol B0 solution. To do this, they should check for possible color changes and/or a possible precipitation, insoluble complexes, or crystals.
Before adding a medication, verify if it is soluble and stable in water within the pH limits of Hemosol B0 (the pH range of the reconstituted solution is 7.0 to 8.5). Additives may not be compatible. The instructions for use of the medication to be added should be consulted.
Eliminate any liquid from the injection port, hold the bag in a vertical downward position, add the medication through the injection port, and mix completely. The solution must be administered immediately. The introduction and mixing of additives should always be performed before connecting the solution bag to the extracorporeal circuit.
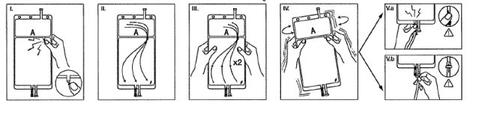
IRemove the bag wrapper immediately before use and discard other packaging materials. Open the seal by breaking the breakable rod located between the two compartments of the bag. The rod will remain inside the bag (see figure I, below).
IIEnsure that all liquid from the small compartment A passes to the large compartment B (see figure II, below).
IIIClarify twicethe small compartment A by forcing the mixed solution to return to this compartment and then again to the large compartment B (see figure III, below).
IVOnce the small compartment A is empty, shake the large compartment B to completely mix its contents. The solution is now ready to use, and the bag can be hung on the equipment (see figure IV, below).
VThe dialysis or substitution fluid line can be connected to either of the two access ports.
V.aIf the luer access is used, remove the plug and connect the male luer lock connector of the dialysis or substitution fluid line to the female luer receptor of the bag; do it firmly. Break the colored breakable rod at its base with your fingers, and move it back and forth. Do not use tools. Check that the rod is completely separated and that the fluid circulates freely. The rod will remain in the luer port during treatment (see figure V.a, below)
V.bIf the injection port is used, first remove the plug by lifting it. The injection port is a port that can be disinfected with a swab. Then introduce the spike through the rubber wall. Check that the fluid circulates freely (see figure V.b, below)
The solution must be used immediately after removing the wrapper. If not used immediately, the reconstituted solution must be used within 24 hours after the addition of the electrolyte solution to the buffer solution, including the duration of treatment.
The reconstituted solution is for single use. Discard any remaining solution immediately after use.
The disposal of unused medication and all materials that have come into contact with it will be carried out in accordance with local regulations.
-----------------------------------------------------------------------------------------------------------------------
HEMOSOL B0 – POLYOLEFIN WITH LUER CONNECTOR AND VALVE
Composition of Hemosol B0
The active ingredients before and after reconstitution are:
Active ingredients before reconstitution:
1000 ml of solution from the small compartment (A)contain:
Calcium chloride, 2H2O 5.145 g
Magnesium chloride, 6 H2O 2.033 g
Lactic acid 5.4 g
1000 ml of solution from the large compartment (B)contain:
Sodium bicarbonate 3.09 g
Sodium chloride 6.45 g
Active ingredients after reconstitution:
The solutions from compartments A (250 ml) and B (4750 ml) are mixed to produce a reconstituted solution (5000 ml) with the following composition:
mmol/l
Calcium, Ca2+ 1.75
Magnesium, Mg2+ 0.5
Sodium, Na+ 140
Chloride Cl- 109.5
Lactate 3
Bicarbonate, HCO3- 32
Theoretical osmolality: 287 mOsm/l
Other components are:carbon dioxide (E-290) and water for injectable preparations.
Appearance of the product and package contents
Hemosol B0 is presented in a two-compartment bag. The bag is covered by a transparent wrapper.
The final reconstituted solution is obtained after breaking the peelable seal and mixing both solutions.
The reconstituted solution is transparent and colorless. Each bag (A+B) contains 5000 ml of solution for hemofiltration, hemodiafiltration, and/or hemodialysis.
Each box contains two bags and a leaflet.
Marketing authorization holder:
Vantive Belgium SRL
Boulevard d´Angleterre 2
1420 Braine-l´Alleud
Belgium
Manufacturer:
Bieffe Medital S.P.A.
Via Stelvio 94,
23035 Sondalo (SO),
Italy
or
Vantive Manufacturing Limited
Moneen Road,
Castlebar, Co. Mayo F23 XR63
Ireland
Further information about this medicinal product can be obtained from the local representative of the marketing authorization holder:
Vantive Health, S.L.
Polígono industrial sector 14
C/ Pouet de Camilo nº2
46394 Ribarroja del Turia
Valencia
Spain
This medicinal product is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Germany, Austria, Belgium, Bulgaria, Croatia, Cyprus, Denmark, Slovakia, Slovenia, Spain, Estonia, Finland, France, Greece, Iceland, Ireland, Latvia, Lithuania, Luxembourg, Malta, Norway, Netherlands, Poland, Portugal, United Kingdom (Northern Ireland), Czech Republic, and Sweden: Hemosol B0.
Date of last revision of this leaflet: 11/2018
__________________________________________________________________________
This information is intended for healthcare professionals only
Hemosol B0 solution for hemodialysis and hemofiltration
Precautions
Follow the instructions for use and handling of Hemosol B0 accurately.
The solutions from the two compartments mustbe mixed before use.The use of a contaminated hemofiltration solution can produce sepsis, shock, and fatal disorders.
To increase patient comfort, Hemosol B0 can be heated to 37°C. Preheating before using the solution should be done before reconstitution using only dry heat. The solutions should not be heated in water or in a microwave oven. Before administration and whenever the solution and container allow, it should be visually checked that the solution does not contain particles or has lost its original color. Do not administer the solution unless it is transparent and the seal is intact.
The addition of sodium bicarbonate may increase the risk of metabolic alkalosis.
Before and during treatment, the electrolyte and acid-base balance should be carefully monitored. Since Hemosol B0 does not contain potassium, the potassium concentration in the serum should be monitored before and after hemofiltration and/or hemodialysis. Potassium addition may be necessary.
Up to 1.2 mmol/l of phosphate can be added to the solution. If potassium phosphate is added, the total potassium concentration should not exceed 4 mEq/l (4 mmol/l).
The volume and rate at which Hemosol B0 is used will depend on the electrolyte concentration in the blood, acid-base balance, and overall clinical condition. The administration schedule (dose, perfusion rate, and cumulative volume) of Hemosol B0 should be determined by a physician. Continuous hemofiltration will eliminate excess fluids and electrolytes.
In case of fluid imbalance, the clinical situation should be closely monitored and fluid balance corrected as necessary.
Overdose will result in fluid overload in patients with renal failure, which could have serious consequences, such as congestive heart failure or alterations in acid-base or electrolyte balance.
The solution does not contain glucose, so its administration may cause hypoglycemia. Blood glucose levels should be regularly monitored.
Hemosol B0 contains bicarbonate and lactate (a bicarbonate precursor), which can affect the patient's acid-base balance. If metabolic alkalosis appears or worsens during treatment with the solution, it may be necessary to reduce the perfusion rate or suspend administration.
Posology
The commonly used flow rates for the substitution solution in hemofiltration and hemodiafiltration are:
Adults: 500-3000 ml/hour
The commonly used flow rates for the dialysis solution (dialysate) in continuous hemodialysis are:
Adults: 500-2500 ml/hour
The commonly used flow rates in adults are between 2000 and 2500 ml/hour, which corresponds to a daily fluid volume of approximately 48 to 60 L.
Pediatric population
The flow rate range for the substitution solution in hemofiltration and hemodiafiltration and for the dialysis solution (dialysate) in continuous hemodialysis is:
Children (from neonates to adolescents up to 18 years): 1000 to 2000 ml/h/1.73 m2.
Flow rates up to 4000 ml/h/1.73 m2 may be necessary, especially in younger children (≤ 10 kg). In general, the absolute flow rate (in ml/h) in the pediatric population should not exceed the maximum flow rate for adults.
Instructions for use and handling
The electrolyte solution (small compartment A) is added to the buffer solution (large compartment B) after breaking the peelable seal immediately before use to obtain the reconstituted solution.
Use only with suitable extracorporeal renal replacement equipment.
Aseptic technique should be used throughout the handling and administration process to the patient.
Use the solution if the wrapper is not damaged, all seals are intact, the peelable seal is not broken, and the solution is transparent. Squeeze the bag to ensure there are no leaks. If a leak is found, discard the solution immediately as its sterility cannot be guaranteed.
The large compartment B has an injection port to add other medications that may be necessary once the solution is reconstituted.
It is the responsibility of the physician to judge the compatibility of an added medication to the Hemosol B0 solution. To do this, they should check for possible color changes and/or a possible precipitation, insoluble complexes, or crystals.
Before adding a medication, verify if it is soluble and stable in water within the pH limits of Hemosol B0 (the pH range of the reconstituted solution is 7.0 to 8.5). Additives may not be compatible. The instructions for use of the medication to be added should be consulted.
Eliminate any liquid from the injection port, hold the bag in a vertical downward position, add the medication through the injection port, and mix completely. The solution must be administered immediately. The introduction and mixing of additives should always be performed before connecting the solution bag to the extracorporeal circuit.
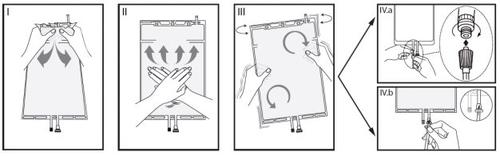
IRemove the bag wrapper immediately before use and discard other packaging materials. Open the seal by holding the small compartment between the two hands and squeezing until an opening is created in the peelable seal between the two compartments (see figure I, below).
IIPress the large compartment with both hands until the peelable seal between the two compartments is fully open (see figure II, below).
IIIEnsure that the solutions are completely mixed by gently shaking the bag. The solution is now ready to use, and the bag can be hung on the equipment (see figure III, below).
IVThe dialysis or substitution fluid line can be connected to either of the two access ports.
IV.aIf the luer connector is used, remove the plug with a twisting and pulling motion and connect the male luer lock connector of the dialysis or substitution fluid line to the female luer receptor of the bag with a pushing and twisting motion. Ensure that the connection is secure and tight. The connector will open. Check that the fluid circulates freely (see figure IV.a, below).
If the dialysis or substitution fluid line is disconnected from the luer connector, the connector will close, and the solution flow will stop. The luer port is a needle-free port that can be cleaned.
IV.bIf the injection port is used, first remove the plug by lifting it. The injection port is a port that can be disinfected with a swab. Then introduce the spike through the rubber wall. Check that the fluid circulates freely (see figure IV.b, below).
The solution must be used immediately after removing the wrapper. If not used immediately, the reconstituted solution must be used within 24 hours after the addition of the electrolyte solution to the buffer solution, including the duration of treatment.
The reconstituted solution is for single use. Discard any remaining solution immediately after use.
The disposal of unused medication and all materials that have come into contact with it will be carried out in accordance with local regulations.
- Country of registration
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to HEMOSOL B0 SOLUTION FOR HEMOFILTRATION AND HEMODIALYSISDosage form: HEMOFILTRATION, 2 mmol potassium/lActive substance: HemofiltratesManufacturer: Nikkiso BelgiumPrescription requiredDosage form: HEMOFILTRATION, 4 mmol potassium/lActive substance: HemofiltratesManufacturer: Nikkiso BelgiumPrescription requiredDosage form: HEMOFILTRATION, -Active substance: HemofiltratesManufacturer: Nikkiso BelgiumPrescription required
Online doctors for HEMOSOL B0 SOLUTION FOR HEMOFILTRATION AND HEMODIALYSIS
Discuss questions about HEMOSOL B0 SOLUTION FOR HEMOFILTRATION AND HEMODIALYSIS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















