HEMLIBRA 150 mg/mL Injectable Solution


How to use HEMLIBRA 150 mg/mL Injectable Solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Hemlibra 150mg/ml solution for injection
emicizumab
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
In addition to this leaflet, your doctor will give you a patient information card that contains important safety information you need to know. Keep this card with you.
Contents of the package leaflet
- What is Hemlibra and what is it used for
- What you need to know before you use Hemlibra
- How to use Hemlibra
- Possible side effects
- Storage of Hemlibra
- Contents of the pack and other information
- Instructions for use
1. What is Hemlibra and what is it used for
What is Hemlibra
Hemlibra contains the active substance “emicizumab”. This belongs to a group of medicines called “monoclonal antibodies”. Monoclonal antibodies are a type of protein that recognizes and binds to a target in the body.
What Hemlibra is used for
Hemlibra is a medicine that can be used to treat patients of all ages with hemophilia A (congenital factor VIII deficiency):
- who have developed factor VIII inhibitors
- who have not developed factor VIII inhibitors with:
- severe disease (factor VIII level in blood is less than 1%)
- moderate disease (factor VIII level in blood is 1% to 5%) with a severe bleeding phenotype.
Hemophilia A is a hereditary condition caused by the lack of factor VIII, a substance essential for blood to form clots and to stop any bleeding.
The medicine prevents bleeding or reduces bleeding episodes in people with this condition.
Some patients with hemophilia A may develop factor VIII inhibitors (antibodies against factor VIII) that prevent replacement factor VIII from working.
How Hemlibra works
Hemlibra restores the function of activated factor VIII that is lacking and necessary for effective blood coagulation. Its structure is different from factor VIII, so Hemlibra is not affected by factor VIII inhibitors.
2. What you need to know before you use Hemlibra
Do not use Hemlibra
- if you are allergic to emicizumab or any of the other ingredients of this medicine (listed in section 6). If you are not sure, talk to your doctor, pharmacist, or nurse before using Hemlibra.
Warnings and precautions
Before you start using Hemlibra, it is very important that you talk to your doctor about the use of “bypassing agents”(medicines that help blood to clot, but work in a different way to factor VIII). This is because you may need to change your treatment with bypassing agents while receiving Hemlibra. Examples of bypassing agents are activated prothrombin complex concentrate (aPCC) and recombinant factor VIIa (rFVIIa). Serious and potentially life-threatening side effects can occur when aPCC is used in patients who are also receiving Hemlibra: Potentially serious side effects of using aPCC while receiving Hemlibra.
- Destruction of red blood cells (thrombotic microangiopathy)
- This is a serious and potentially life-threatening condition.
- When a person has this condition, the lining of the blood vessels can be damaged and blood clots can form in the small blood vessels. In some cases, this can cause damage to the kidneys or other organs.
- You should be cautious if you are at high risk of having this condition (you have had this condition in the past, or a family member has had it), or if you are taking medicines that may increase the risk of developing this condition, such as cyclosporin, quinine, and tacrolimus.
- It is important to know the symptoms of thrombotic microangiopathy in case you develop the condition (see section 4, “Possible side effects” for a list of symptoms).
Stop using Hemlibra and aPCC and talk to your doctor immediatelyif you or your caregiver notice any symptoms of thrombotic microangiopathy.
- Blood clots (thromboembolism)
- In rare cases, a blood clot can form inside the blood vessels and block them, and could be potentially life-threatening.
- It is important to know the symptoms of such internal blood clots in case they form (see section 4, “Possible side effects” for a list of symptoms).
Stop using Hemlibra and aPCC and talk to your doctor immediatelyif you or your caregiver notice any symptoms of blood clots in the blood vessels.
Other important information about Hemlibra
- Formation of antibodies (immunogenicity)
- You may notice that your bleeding is not being controlled with the prescribed dose of this medicine. This may be due to the development of antibodies against this medicine.
Talk to your doctor immediatelyif you or your caregiver notice an increase in bleeding. Your doctor may decide to change your treatment if this medicine stops working for you.
Children under 1 year of age
In children under 1 year of age, the blood system is still developing. If your child is under 1 year of age, your doctor may prescribe Hemlibra only after carefully weighing the expected benefits and risks of using Hemlibra.
Other medicines and Hemlibra
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
- Use of a bypassing agent while receiving Hemlibra
- Before you start using Hemlibra, talk to your doctor and follow their instructions carefully about when to use a bypassing agent and the dose and schedule to follow. Hemlibra increases the blood's ability to clot. Therefore, the dose of the bypassing agent needed may be lower than the dose you used before starting Hemlibra.
- Use aPCC only if you cannot use another treatment. If aPCC is needed, talk to your doctor if you think you need more than 50 units/kg of aPCC in total. For more information on the use of aPCC while receiving Hemlibra, see section 2: Potentially serious side effects of using aPCC while receiving Hemlibra.
- Despite limited experience with the concomitant administration of antifibrinolytics with aPCC or rFVIIa in patients treated with Hemlibra, you should be aware that thrombotic events may occur when antifibrinolytics are administered intravenously in combination with aPCC or rFVIIa.
Lab tests
Talk to your doctor if you use Hemlibra before having lab tests to measure your blood's ability to clot. This is because the presence of Hemlibra in your blood may interfere with some of these lab tests and give incorrect results.
Pregnancy and breastfeeding
- You must use an effective method of birth control (contraception) during treatment with Hemlibra and for 6 months after the last injection of Hemlibra.
- If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, talk to your doctor or pharmacist before using this medicine. Your doctor will weigh the benefits of you taking Hemlibra against the risks to your baby.
Driving and using machines
It is unlikely that this medicine will affect your ability to drive or use machines.
3. How to use Hemlibra
Hemlibra is supplied in single-use vials as a ready-to-use solution that does not need to be diluted.
A qualified doctor who is experienced in treating patients with hemophilia will teach you how to use Hemlibra. Follow your doctor's instructions for administering this medicine exactly. If you are unsure, talk to your doctor again.
Keep a record
Each time you use Hemlibra, write down the name and batch number of the medicine.
How much Hemlibra to use
The dose of Hemlibra depends on your weight, and your doctor will calculate the amount (in mg) and the corresponding amount of Hemlibra solution (in ml) to be injected:
- Loading dose regimen, weeks 1-4: the dose is 3 milligrams per kilogram of body weight, injected once a week.
- Maintenance dose regimen, week 5 and later: the dose is either 1.5 milligrams per kilogram of body weight, injected once a week; 3 milligrams per kilogram of body weight, injected every 2 weeks; or 6 milligrams per kilogram of body weight, injected every 4 weeks.
The decision to use the maintenance dose of 1.5 mg/kg once a week, 3 mg/kg every 2 weeks, or 6 mg/kg every 4 weeks should be discussed with your doctor and, when applicable, your caregiver.
Do notmix different concentrations of Hemlibra (30 mg/ml and 150 mg/ml) in a single injection to achieve the total volume to be administered.
Do not administer more than 2 ml of Hemlibra solution in a single injection.
How Hemlibra is administered
If you or your caregiver administer an injection of Hemlibra, you must read and follow the instructions in section 7, “Instructions for use” carefully.
- Hemlibra is administered by injection under the skin (subcutaneously).
- Your doctor or nurse will teach you how to inject Hemlibra.
- Once you have received training, you can inject the medicine yourself at home, either alone or with the help of a caregiver.
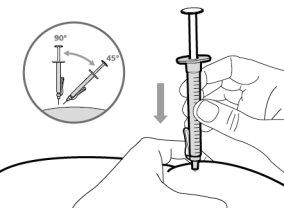
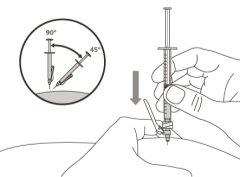
- To correctly insert the needle under the skin, make a skin fold at the injection site with your free hand. It is important to make a skin fold to ensure that you inject under the skin (into the fatty tissue) and not deeper (into the muscle). An injection into the muscle can be painful.
- Prepare and administer the injection in a clean and germ-free environment using an aseptic technique. Your doctor or nurse will give you more information about this.
Where to inject Hemlibra
- Your doctor will teach you which areas of the body are suitable for injecting Hemlibra.
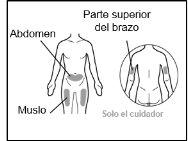
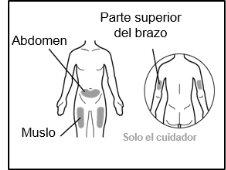
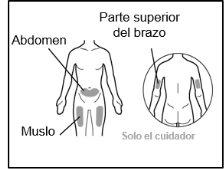
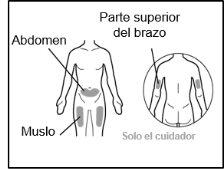
- Recommended injection sites are: the front of the waist (lower abdomen), the upper outer arm, or the front of the thighs. Only use recommended injection sites.
- For each injection, use a different area of the body than you used for the previous injection.
- Do not administer injections in areas where the skin is red, bruised, tender, hardened, or has moles or scars.
- When using Hemlibra, other medicines injected under the skin should be administered in a different area.
Use of syringes and needles
- To withdraw the Hemlibra solution from the vial, transfer it to a syringe, and inject it under the skin, you need a syringe, a transfer needle with a 5-micron filter, or a vial adapter with a 5-micron filter and an injection needle.
- Syringes, transfer needles with filters, or vial adapters with filters and injection needles are not included in the pack. For more information, see section 6, “What you need to administer Hemlibra that is not included in the pack”.
- Make sure to use a new injection needle for each injection and discard it after a single use.
- For an injection of up to 1 ml of Hemlibra solution, use a 1-ml syringe.
- For an injection of more than 1 ml and up to 2 ml of Hemlibra solution, use a 2-3 ml syringe.
Use in children and adolescents
Hemlibra can be used in children and adolescents of all ages.
- A child may inject the medicine themselves, provided that the healthcare professional and the child's parent or caregiver agree. Self-injection is not recommended in children under 7 years of age.
If you use more Hemlibra than you should
If you use more Hemlibra than you should, talk to your doctor immediately. This is because you may be at risk of experiencing side effects such as blood clots. Follow your doctor's instructions for administering Hemlibra exactly. If you are unsure, talk to your doctor, pharmacist, or nurse again.
If you forget to use Hemlibra
- If you miss a scheduled injection, inject the missed dose as soon as possible before the next scheduled dose. Then, continue with the scheduled injections of the medicine. Do not inject two doses on the same day to make up for missed doses.
- If you are unsure, talk to your doctor, pharmacist, or nurse.
If you stop using Hemlibra
Do not stop using Hemlibra without talking to your doctor. If you stop using Hemlibra, you may no longer be protected against bleeding.
If you have any other questions about using this medicine, talk to your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects of using aPCC while receiving Hemlibra
Stop using Hemlibra and aPCC and talk to your doctor immediatelyif you or your caregiver notice any of the following side effects:
- Destruction of red blood cells (thrombotic microangiopathy):
- confusion, weakness, swelling of arms and legs, yellowing of the skin and eyes, abdominal pain or back pain, feeling sick (nausea), being sick (vomiting), or urinating less than usual: these symptoms could be signs of thrombotic microangiopathy.
- Blood clots (thromboembolism):
- swelling, warmth, pain, or redness: these symptoms could be signs of a blood clot in a vein near the surface of the skin.
- headache, numbness of the face, pain or swelling in the eyes, or problems with your vision: these symptoms could be signs of a blood clot in a vein behind the eye.
- discoloration of the skin: this symptom could be a sign of serious damage to the skin tissue.
Other side effects of using Hemlibra
Very common:may affect more than 1 in 10 people
- reaction at the injection site (redness, itching, pain)
- headache
- joint pain
Common:may affect up to 1 in 10 people
- fever
- muscle pain
- diarrhea
- itchy rash or hives (urticaria)
- skin rash
Uncommon:may affect up to 1 in 100 people
- destruction of red blood cells (thrombotic microangiopathy)
- blood clot in a vein behind the eye (cavernous sinus thrombosis)
- serious damage to the skin tissue (necrotic skin lesion)
- blood clot in a vein near the surface of the skin (superficial thrombophlebitis)
- swelling of the face, tongue, and/or throat, and/or difficulty swallowing, or hives, along with difficulty breathing, which are indicative of angioedema
- lack of effect or reduced response to treatment
Reporting side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Hemlibra
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and vial after “EXP”. The expiry date is the last day of the month shown.
Store in a refrigerator (2°C - 8°C). Do not freeze.
Store in the original package to protect from light.
Once removed from the refrigerator, unopened vials can be stored at room temperature (below 30°C) for a maximum of 7 days. After storage at room temperature, unopened vials can be returned to the refrigerator. The total storage time at room temperature should not exceed 7 days.
Discard vials that have been stored at room temperature for more than 7 days or have been exposed to temperatures above 30°C.
Once the solution is transferred from the vial to the syringe, use Hemlibra immediately. Do not refrigerate the solution in the syringe.
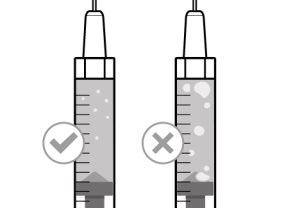
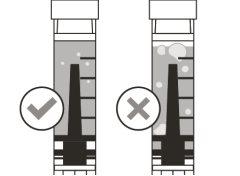
Before using the medicine, check that the solution does not contain particles or has changed color. The solution should be colorless to slightly yellow. Do not use this medicine if it is cloudy, has changed color, or contains visible particles.
Dispose of unused solution properly. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Hemlibra Composition
- The active ingredient is emicizumab. Each vial of Hemlibra contains 60 mg (0.4 ml at a concentration of 150 mg/ml), 105 mg (0.7 ml at a concentration of 150 mg/ml), 150 mg (1 ml at a concentration of 150 mg/ml), or 300 mg (2 ml at a concentration of 150 mg/ml) of emicizumab.
- The other components are L-arginine, L-histidine, L-aspartic acid, poloxamer 188, and water for injectable preparations.
Appearance of Hemlibra and Container Contents
Hemlibra is an injectable solution. It is a colorless or slightly yellowish liquid.
Each Hemlibra container contains 1 glass vial.
Only some package sizes may be marketed.
What is Needed for Hemlibra Administration Not Included in the Container
To extract the Hemlibra solution from the vial, insert it into a syringe, and inject it under the skin, a syringe, a transfer needle with a filter or a vial adapter with a filter, and an injection needle are needed (see section 7, "Instructions for Use").
Syringes
- 1 ml Syringe:transparent polypropylene or polycarbonate syringe with Luer-lock tip, graduated in 0.01 ml or
- 2-3 ml Syringe:transparent polypropylene or polycarbonate syringe with Luer-lock tip, graduated in 0.1 ml.
Note: Low Dead Space (LDS) syringes should be used when using the vial adapter with a filter.
Transfer Devices and Needles
- Transfer Needle with Filter:stainless steel with Luer-lock connection, 18 G gauge, 35 mm (1½″) length, containing a 5-micron filter and preferably with a semi-blunt tip or
- Vial Adapter with Filter:polypropylene with Luer-lock connection, with an integrated 5-micron filter, and an outer diameter adjustment of the vial neck of 15 mm, and
- Injection Needle:stainless steel with Luer-lock connection, 26 G gauge (acceptable range: 25-27 gauge), preferred length of 9 mm (3/8″) or up to 13 mm (½″), preferably with needle safety.
Marketing Authorization Holder
Roche Registration GmbH
Emil-Barell-Strasse 1
79639 Grenzach-Wyhlen
Germany
Manufacturer
Roche Pharma AG
Emil-Barell-Strasse 1
79639 Grenzach-Wyhlen
Germany
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
|
Ceská republika Roche s. r. O. Tel.: +420 - 2 20382111 | Magyarország Roche (Magyarország) Kft. Tel: +36 – 1 279 4500 |
Danmark Roche Pharmaceuticals A/S Tel.: +45 - 36 39 99 99 | Malta (See Ireland) |
Deutschland Roche Pharma AG Tel: +49 (0) 7624 140 | Nederland Roche Nederland B.V. Tel: +31 (0) 348 438050 |
Eesti Roche Eesti OÜ Tel: + 372 - 6 177 380 | Norge Roche Norge AS Tlf: +47 - 22 78 90 00 |
Ελλάδα Roche (Hellas) A.E. Τηλ: +30 210 61 66 100 | Österreich Roche Austria GmbH Tel: +43 (0) 1 27739 |
España Roche Farma S.A. Tel: +34 - 91 324 81 00 | Polska Roche Polska Sp.z o.o. Tel: +48 - 22 345 18 88 |
France Roche Tél: +33 (0) 1 47 61 40 00 | Portugal Roche Farmacêutica Química, Lda Tel: +351 - 21 425 70 00 |
Hrvatska Roche d.o.o. Tel: +385 1 4722 333 Ireland Roche Products (Ireland) Ltd. Tel: +353 (0) 1 469 0700 | România Roche România S.R.L. Tel: +40 21 206 47 01 Slovenija Roche farmacevtska družba d.o.o. Tel: +386 - 1 360 26 00 |
Ísland Roche Pharmaceuticals A/S c/o Icepharma hf Sími: +354 540 8000 | Slovenská republika Roche Slovensko, s.r.o. Tel: +421 - 2 52638201 |
Italia Roche S.p.A. Tel: +39 - 039 2471 | Suomi/Finland Roche Oy Puh/Tel: +358 (0) 10 554 500 |
Κύπρος Γ.Α.Σταμάτης & Σια Λτδ. Τηλ: +357 - 22 76 62 76 | Sverige Roche AB Tel: +46 (0) 8 726 1200 |
Latvija Roche Latvija SIA Tel: +371 - 6 7039831 | United Kingdom (Northern Ireland) Roche Products (Ireland) Ltd. Tel: +44 (0) 1707 366000 |
Date of Last Revision of this Leaflet
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu/
- Instructions for Use
Transfer Needle with Filter Option (for transferring the medication from the vial to the syringe) |
Instructions for Use
Hemlibra
Injection
Single-Dose Vial(s)
You must read, understand, and follow the instructions for use before injecting Hemlibra. Your healthcare professional should teach you how to prepare, measure, and inject Hemlibra correctly before you use it for the first time. If you are unsure, consult your healthcare professional. Important Information: Do not follow these instructions when using a Vial Adapter to transfer Hemlibra from the vial. These instructions are only used with the Transfer Needle ? Do not inject yourself or another person unless your healthcare professional has taught you how to do it. ? Check that the name Hemlibra appears on the box and the vial label. ? Before opening the vial, read the vial label to check that you have the medication of the correct concentration to administer the prescribed dose for you. You may need to use more than 1 vial to administer the correct total dose. ? Check the expiration date on the box and the vial label. Do notuse it after the expiration date. ? Onlyuse the vial once.Once the dose is injected, discard the remaining Hemlibra solution in the vial. Do not store unused medication in the vial for later use. ? Onlyuse the syringes, transfer needles, and injection needles prescribed by your healthcare professional. ? Only usethe syringes, transfer needles, and injection needles once. Discard the caps, vial(s), and used syringes and needles. ? If the prescribed dose is more than 2 ml, you will need to administer more than one subcutaneous injection of Hemlibra; contact your healthcare professional for proper injection instructions. ? You must inject Hemlibra only under the skin. Storage of Hemlibra Vials: ? Store the vial in the refrigerator (2 ºC to 8 ºC). Do notfreeze.
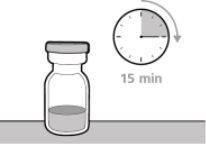
? Discard vials that have been stored at room temperature for more than 7 days or have been at a temperature above 30°C. ? Keep vials out of sight and reach of children. ? Remove the vial from the refrigerator 15 minutes before use and let it reach room temperature (below 30 ºC) before preparing an injection.
Storage of Needles and Syringes: ? Keep the transfer needle, injection needle, and syringe dry.
Inspection of Medication and Materials: ? Gather all the materials mentioned below to prepare and administer the injection. ? Checkthe expiration date on the box, vial label, and the materials mentioned below. Do not usethem after the expiration date. ? Do not usethe vial if:
? Inspect the materials for damage. Do not use them if they appear damaged or have fallen to the ground. ? Place the materials on a flat, clean, and well-lit work surface.
For injection, you can use: ? The thigh (front and middle). ? The stomach area (abdomen), except for the 5 cm around the navel. ? The outer area of the upper arm (only if the caregiver administers the injection). ? You must use a different injection site each time you apply an injection, at least 2.5 cm away from the one used in any previous injection. ? Do notinject into areas that may be irritated by a belt or band.
Preparing the Syringe for Injection: ? When you have filled the syringe with the medication, you must use it immediately. ? Once the injection needle cap is removed, the medication must be injected under the skin within 5 minutes.
Important Information After Injection: ? If you notice blood drops at the injection site, you can press it with a sterile cotton swab or gauze for at least 10 seconds until the bleeding stops. ? If you have a hematoma (a small bleed under the skin), you can also apply ice with gentle pressure. If the bleeding does not stop, contact a healthcare professional. ? Do notrub the injection site after injection. Disposal of Medication and Materials: Important: Always keep the sharp object container out of the reach of children. Discard any used caps, vials, needles, and syringes in a sharp object container.
? If you do not have a sharp object container, you can use a household trash container that:
? When the sharp object container is almost full, you must follow local guidelines for proper disposal. ? Do notdiscard any used sharp object container in household trash unless local guidelines permit it. Do not recycleyour sharp object container.
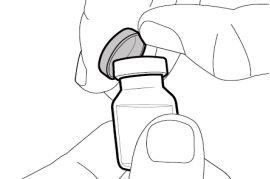
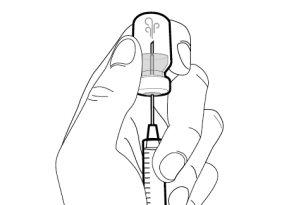
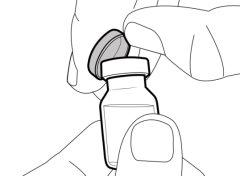
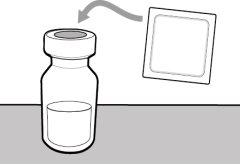
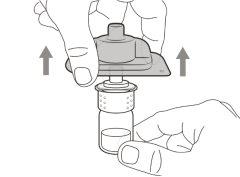
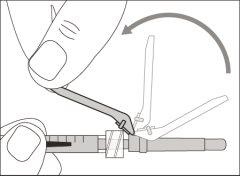
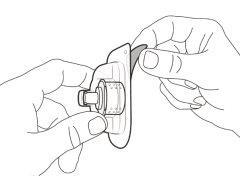
Step 1. Remove the Vial Cap and Clean the Top
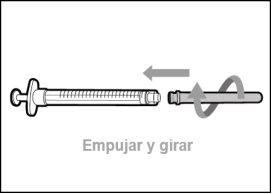
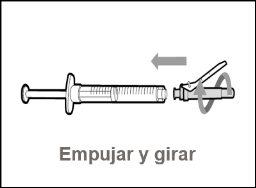
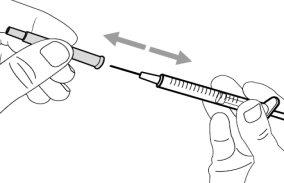
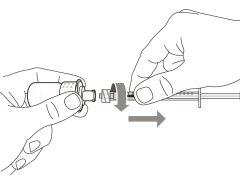
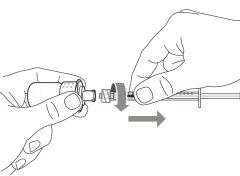
Step 2. Attach the Transfer Needle with Filter to the Syringe
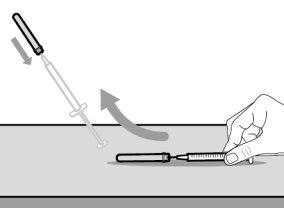
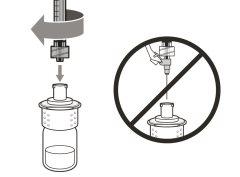
Step 3. Remove the Transfer Needle Cap
|
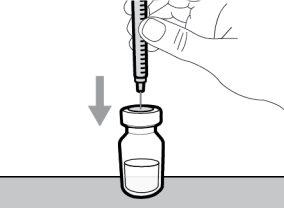
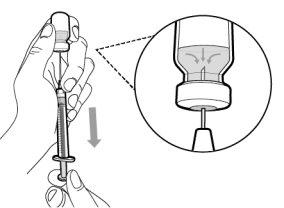
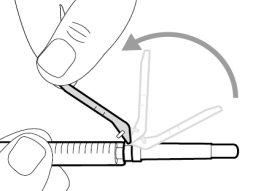
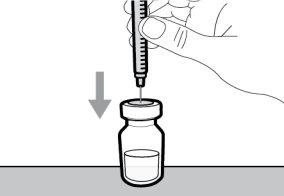
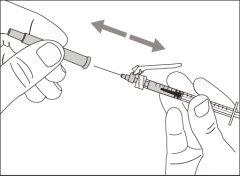
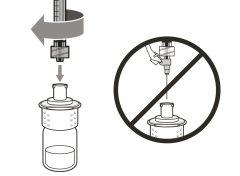
Step 4. Inject air into the vial
|
|
|
|
|
|
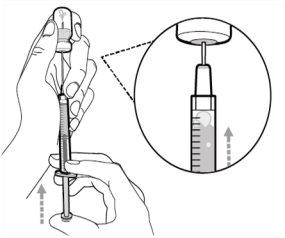
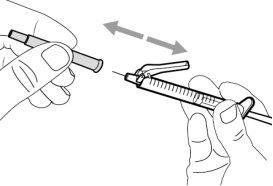
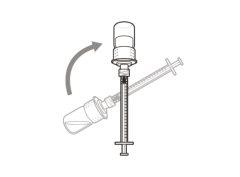
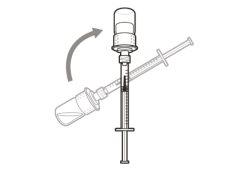
Step 5. Transfer the medication to the
syringe
|
|
Important:If the prescribed dose is greater than the amount of Hemlibra in the vial, withdraw all the medicationand now refer to the “Combining vials”section.
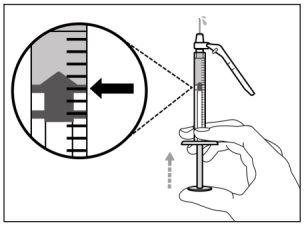
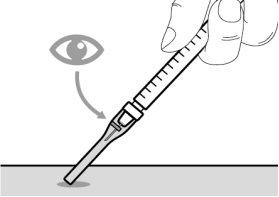
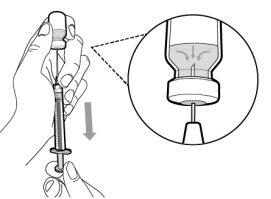
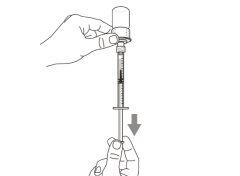
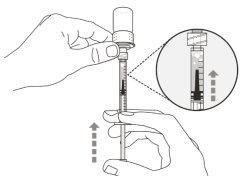
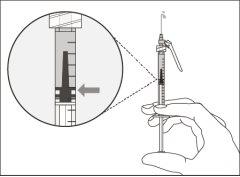
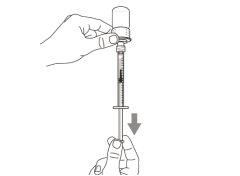
Step 6. Remove air bubbles
|
|
Note:Make sure you have enough medication in the syringe to complete the dose before proceeding to the next step. If you cannot withdraw all the medication, turn the vial upside down to reach the remaining amount.
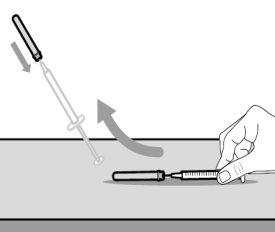
Do notuse the transfer needle to inject the medication, as it may cause pain and bleeding.
- INJECTION
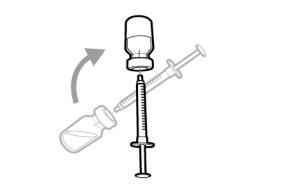
Step 7. Replace the transfer needle cap
|
|
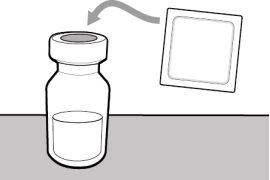
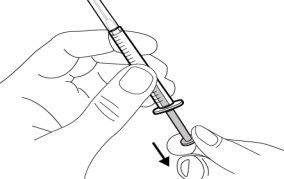
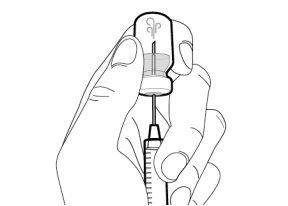
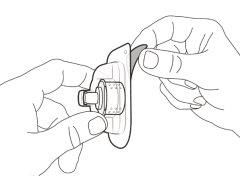
Step 8. Clean the injection site
|
|
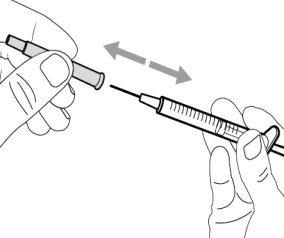
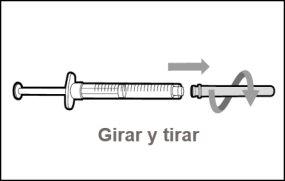
Step 9. Remove the used transfer needle from the syringe
|
|
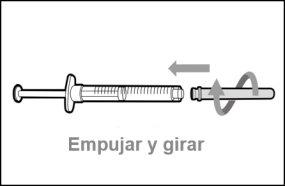
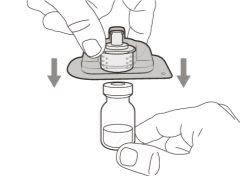
Step 10. Attach the injection needle to the syringe
|
|
Step 11. Remove the safety protector
|
|
Step 12. Remove the injection needle cap
|
|
Step 13. Adjust the plunger according to the prescribed dose
|
|
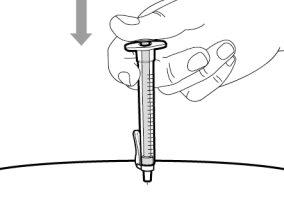
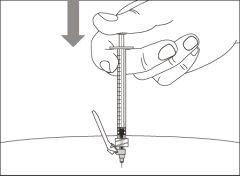
Step 14. Subcutaneous injection (under the skin)
|
|
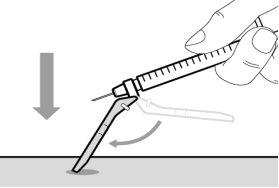
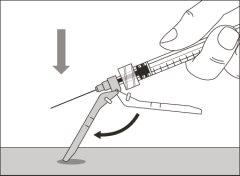
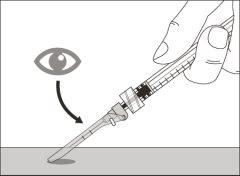
Step 15. Inject the medication
|
|
- DISPOSAL OF THE SYRINGE AND NEEDLE
Step 16. Cover the needle with the safety protector
|
|
|
|
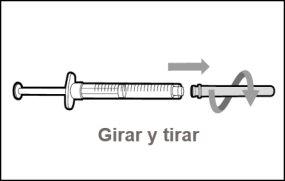
Step 17. Discard the syringe and needle.
|
|
Combining vials |
If you need to use more than 1 vial to reach the prescribed dose, follow these steps after withdrawing the medication from the first vial as described in step 5. You must use a new transfer needle for each vial. |
Step A. Replace the transfer needle cap
|
|
Step B. Remove the used transfer needle from the syringe
|
|
Step C. Attach a new transfer needle with a filter to the syringe
|
|
Step D. Remove the transfer needle cap
|
|
Step E. Inject air into the vial
|
|
|
|
|
|
Step F. Transfer the medication to the syringe
|
|
Note: Make sure you have enough medication in the syringe to complete the dose before proceeding to the next steps. If you cannot withdraw all the medication, turn the vial upside down to reach the remaining amount. |
Do notuse the transfer needle to inject the medication, as it may cause damage such as pain and bleeding.
Repeat steps A to F with each additional vial until you have more than the amount of medication needed for your prescribed dose. When you have finished, keep the transfer needle inserted in the vial and return to step 6 “Remove air bubbles”. Continue with the remaining steps. |
Vial adapter with filter Option (for transferring medication from the vial to the syringe)
|
Instructions for Use Hemlibra Injection Single-dose vial(s) |
You must read, understand, and follow the Instructions for Use before injecting Hemlibra. Your healthcare professional should teach you how to prepare, measure, and inject Hemlibra correctly before you use it for the first time. If you have any questions, consult your healthcare professional. |
Important information: |
Do not follow these instructions when using a transfer needle to transfer Hemlibra from the vial. These instructions are only used with the vial adapter. |
|
Storage of Hemlibra vials:
|
Storage of vial adapters, needles, and syringes:
- Keep the vial adapter, injection needle, and syringe dry.
- Keep the vial adapter, injection needle, and syringe out of sight and reach of children.
Inspection of the medicine and materials:
- Gather all the materials mentioned below to prepare and administer the injection.
- Checkthe expiration date on the box, on the vial label, and on the materials mentioned below. Do not usethem after the expiration date.
- Do not usethe vial if:
- the medicine is cloudy, blurry, or has changed color
- the medicine contains particles.
- the cap covering the stopper is missing.
- Inspect the materials for damage. Do not usethem if they appear damaged or have fallen to the floor.
- Place the materials on a flat, clean, and well-lit work surface.
The box includes:
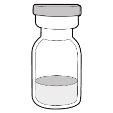
- Vial with the medicine

- Hemlibra instructions for use
The box does not include:

- Alcohol wipesNote:if you need to use more than 1 vial to inject the prescribed dose, you must use a new alcohol wipe for each vial.
- Gauze
- Cotton
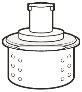
- Vial adapter with filter(to be inserted into the top of the vial). Note:use it to transfer the medicine from the vial to the syringe. If you need more than 1 vial to inject the prescribed dose, you must use a new vial adapter for each vial.
Do not insert the injection needle into the vial adapter.

- Low Dead Space (LDS) syringe with plunger
Important:
- To inject up to 1 ml, use a 1 ml LDS syringe.
- To inject more than 1 ml, use a 2 or 3 ml LDS syringe.
- Note: do not usea 2 or 3 ml LDS syringe for doses up to 1 ml.
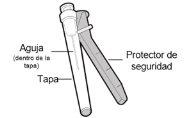
- Injection needle with safety protector (used to inject the medicine)
- Do not insertthe injection needle into the vial adapter or use the injection needle to extract the medicine from the vial.

- Sharps container
Prepare: | |
|
|
Selection and preparation of the injection site: | |
|
|
For injection, you can use:
| |
Preparing the syringe for injection:
| |
Important information after injection:
| |
Disposal of medicine and materials: Important: Always keep the sharps container out of the reach of children.
|
| |
Step 1. Remove the vial cap and clean the top | |
|
|
|
|
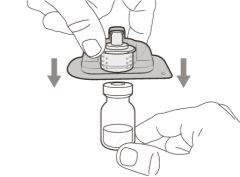
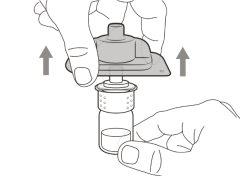
Step 2. Insert the vial adapter into the vial | |
|
Do not removethe vial adapter from the transparent plastic blister. |
|
|
|
|
Step 3. Connect the syringe to the vial adapter | |
|
|
Step 4. Transfer the medicine to the syringe | |
|
|
|
|
Important:if the prescribed dose is more than the amount of Hemlibra in the vial, withdraw all the medicineand consult the “Vial combination”section now. | |
Step 5. Remove air bubbles | |
|
|
|
|
Note:make sure you have enough medicine in the syringe to complete the dose before proceeding to the next step. |
| |
Step 6. Clean the injection site | |
|
|
Step 7. Remove the syringe from the vial adapter | |
|
|
Step 8. Place the injection needle on the syringe | |
|
|
Step 9. Remove the safety protector | |
|
|
Step 10. Uncover the injection needle | |
|
|
Step 11. Adjust the plunger according to the prescribed dose | |
|
|
Step 12. Subcutaneous injection (under the skin) | |
|
|
Step 13. Inject the medicine | |
|
|
| |
Step 14. Cover the needle with the safety protector | |
|
|
|
? Keep your fingers behind the safety protector and away from the needle at all times. ? Do notseparate the injection needle. |
Step 15. Discard the syringe and needle | |
|
|
Vial Combination | |
If you need to use more than 1 vial to reach the prescribed dose, follow these steps after extracting the medication from the first vial as described in step 4. You must use a new vial adapter for each vial. | |
Step A. Insert a new vial adapter into the new vial | |
|
Do not removethe vial adapter from the transparent plastic blister. |
|
|
|
|
Step B. Extract the syringe from the used vial adapter | |
|
|
Step C. Place the syringe on the new vial adapter | |
|
|
Step D. Transfer the medication to the syringe | |
|
|
|
|
Note:Check that you have enough medication in the syringe to complete the dose before proceeding to the next step. | |
Repeat steps A to D with each additional vial until you have more than the amount of medication needed for your prescribed dose. When finished, keep the vial adapter on the vial and return to step 5 “Extract air bubbles”. Continue with the remaining steps. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to HEMLIBRA 150 mg/mL Injectable SolutionDosage form: INJECTABLE, 30 mg/mlActive substance: emicizumabManufacturer: Roche Registration GmbhPrescription requiredDosage form: INJECTABLE, 100 mg/mlActive substance: concizumabManufacturer: Novo Nordisk A/SPrescription requiredDosage form: INJECTABLE, 100 mg/mlActive substance: concizumabManufacturer: Novo Nordisk A/SPrescription required
Online doctors for HEMLIBRA 150 mg/mL Injectable Solution
Discuss questions about HEMLIBRA 150 mg/mL Injectable Solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions