
GEXANA 70 micrograms/hour transdermal patch


How to use GEXANA 70 micrograms/hour transdermal patch
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Gexana70 micrograms/hour transdermal patch EFG
Buprenorphine
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Gexana and what is it used for
- What you need to know before you use Gexana
- How to use Gexana
- Possible side effects
- Storing Gexana
- Contents of the pack and other information
1. What is Gexana and what is it used for
Gexana is a pain reliever (a medicine for pain relief) indicated for the relief of moderate to severe cancer pain and severe pain that does not respond to other types of pain relievers. Gexana acts through the skin. When the transdermal patch is applied to the skin, the active substance buprenorphine passes through the skin into the bloodstream. Buprenorphine is an opioid (a strong pain reliever) that reduces pain by acting on the central nervous system (in specific nerve cells in the spinal cord and brain). The effect of the transdermal patch lasts for up to a maximum of four days. Gexana is not suitable for the treatment of acute (short-term) pain.
2. What you need to know before you use Gexana
Do not useGexana
- if you are allergic to buprenorphine or any of the other ingredients of this medicine (listed in section 6),
- if you are addicted to strong pain relievers (opioids),
- if you have a disease that makes it difficult for you to breathe or if this may occur,
- if you are taking MAO inhibitors (certain medicines for the treatment of depression) or have taken them in the last two weeks before treatment with Gexana (see "Using Gexana with other medicines"),
- in case of myasthenia gravis (a type of severe muscle weakness),
- in case of delirium tremens (confusion and tremors caused by alcohol withdrawal after habitual excessive alcohol consumption or during an episode of high alcohol consumption),
- if you are pregnant.
Gexana should not be used to treat withdrawal syndrome in drug addicts.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Gexana
- if you have recently drunk a lot of alcohol,
- if you have epileptic seizures or convulsions (attacks),
- if you have altered consciousness (feeling of dizziness or fainting) for unknown reasons,
- if you are in shock (a sign may be cold sweat),
- if you have increased intracranial pressure (e.g. after head injury or brain disease) without the possibility of artificial respiration,
- if you have difficulty breathing or are taking other medication that may make you breathe more slowly or weakly (see "Using Gexana with other medicines"),
- if you have liver problems,
- if you have a tendency to abuse medicines or drugs,
- if you have depression or other diseases that are treated with antidepressants.
The use of these medicines together with Gexana may cause serotonin syndrome, a potentially life-threatening disease (see "Using Gexana with other medicines").
Consult your doctor if any of these cases apply to you or have applied in the past.
Also, note the following precautions:
- Some people may become dependent on strong pain relievers such as Gexana when they use them for a long time. These patients may have effects after they stop using them (see "If you stop using Gexana").
- Fever and ambient heat can lead to higher than normal amounts of buprenorphine in the blood. Also, ambient heat can prevent the transdermal patch from sticking properly. Therefore, consult your doctor if you have a fever and avoid exposure to heat sources (e.g. sauna, infrared lamps, electric blankets or hot water bottles).
- Gexana should not be used in people under 18 years of age because there is no experience in this age group to date.
- Sleep-related breathing disorders: Gexana may cause sleep-related breathing disorders such as sleep apnea (pauses in breathing during sleep) and sleep-related hypoxemia (low oxygen levels in the blood). Symptoms may include pauses in breathing during sleep, nighttime awakenings due to difficulty breathing, difficulty maintaining sleep or excessive daytime sleepiness. Contact your doctor if you or someone else observes these symptoms. Your doctor may consider a dose reduction.
Using Gexana with other medicines
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
- Gexana should not be used with MAO inhibitors (certain medicines for the treatment of depression) or if you have taken them in the last two weeks.
- Gexana may cause drowsiness, vomiting, dizziness or make you breathe more slowly or weakly in some patients. These adverse effects may be intensified if you take other medicines that may have the same effects at the same time. These other medicines include other strong pain relievers (opioids), certain sleeping medicines, anesthetics and medicines for the treatment of certain psychological diseases (tranquilizers, antidepressants and neuroleptics).
- If Gexana is used with some medicines, the effect of the transdermal patch may be intensified. These medicines include, for example, certain anti-infectives and antifungals (e.g. those containing erythromycin or ketoconazole) or medicines for HIV (e.g. those containing ritonavir).
- If Gexana is used with other medicines, the effect of the transdermal patch may be reduced. These medicines include, for example, dexamethasone, certain products for the treatment of epilepsy (e.g. those containing carbamazepine or phenytoin) or medicines used for the treatment of tuberculosis (e.g. rifampicin).
Some medicines may increase the effects of Gexana and may occasionally cause very serious reactions. Do not take any other medicine while using Gexana without consulting your doctor first, especially:
- antidepressants such as moclobemide, tranylcypromine, citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, duloxetine, venlafaxine, amitriptyline, doxepin or trimipramine. These medicines may interact with Gexana and you may experience symptoms such as involuntary muscle contractions, including those that control eye movements, agitation, hallucinations, coma, excessive sweating, tremors, exaggerated reflexes, increased muscle tension, body temperature above 38 °C. Contact your doctor if you experience these symptoms.
The concomitant use of Gexana and sedative medicines such as benzodiazepines or related medicines increases the risk of drowsiness, breathing difficulties (respiratory depression), coma and may put your life at risk. Due to this, concomitant use should only be considered when no other treatment is possible.
However, if your doctor prescribes Gexana together with sedative medicines, the dose and duration of concomitant treatment should be limited by your doctor.
Tell your doctor about all sedative medicines you are taking and follow your doctor's dose recommendation. It may be useful to inform friends or relatives so that they are aware of the signs and symptoms indicated above. Consult your doctor if you experience any symptoms.
Using Gexana with food, drinks and alcohol
Do not drink alcohol while using Gexana. Alcohol may intensify certain adverse effects of the transdermal patch and you may not feel well.
Drinking grapefruit juice during treatment may intensify the effects of Gexana.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Pregnancy
There is not enough experience with the use of Gexana in pregnant women to date. Therefore, Gexana should not be used during pregnancy.
Ask your doctor or pharmacist for advice before using any medicine.
Breast-feeding
Buprenorphine, the active substance contained in the transdermal patch, passes into breast milk and inhibits milk production. Therefore, Gexana should not be used during breast-feeding.
Ask your doctor or pharmacist for advice before using any medicine.
Driving and using machines
Gexana may make you feel dizzy, drowsy or have double or blurred vision and may affect your reflexes so that you do not react adequately or quickly enough in case of sudden or unexpected situations.
This applies especially:
? at the start of treatment
? when changing the dose
? when changing from another medicine to this one
? if you are also taking other medicines that act on the brain
? if you drink alcohol
If you are affected, you should not drive or operate machinery while using Gexana. This also applies at the end of treatment with Gexana. Do not drive or operate machinery for at least 24 hours after removing the patch.
In case of doubt, consult your doctor or pharmacist.
3. How to use Gexana
Follow the instructions for administration of this medicine exactly as prescribed by your doctor. In case of doubt, consult your doctor or pharmacist again.
Gexana is available in three strengths:
Gexana 35 micrograms/hour transdermal patch EFG, Gexana 52.5 micrograms/hour transdermal patch EFG and Gexana 70 micrograms/hour transdermal patch EFG.
Your doctor has chosen this Gexana patch as the most suitable for you.
During treatment, your doctor may change the transdermal patch you use to a smaller or larger one if necessary.
The recommended dose is:
Adults
Follow these instructions unless your doctor has given you different instructions.
Apply Gexana (as described below) and change it after four days, at most. To facilitate use, you can change the patch twice a week on fixed days, e.g. "always on Mondays in the morning and Thursdays in the afternoon". To help you remember when to change the patch, note it on the packaging.
If your doctor has told you to take other pain relievers in addition to the transdermal patch, follow your doctor's instructions strictly, otherwise you will not benefit fully from treatment with Gexana.
Use in children and adolescents
Gexana should not be used in people under 18 years of age because there is no experience in this age group to date.
Elderly patients
No dose adjustment is required in elderly patients.
Patients with renal impairment / patients on dialysis
In patients with renal impairment and patients on dialysis, no dose adjustment is necessary.
Patients with hepatic impairment
In patients with hepatic impairment, the intensity and duration of the effect of Gexana may be affected. If you belong to this group of patients, your doctor will monitor you more closely.
Method of administration
Before applying a transdermal patch
- Choose a smooth area of skin without hair on the upper part of your body, preferably under the collarbone on the chest or on the upper back (see adjacent figure). Ask for help if you cannot apply the transdermal patch yourself.
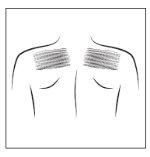
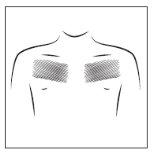
- If the chosen area has hair, cut it with scissors (do not shave it)
- Avoid areas of skin that are red, irritated or have any other type of spots, such as large scars.
- The area of skin you choose must be dry and clean. If necessary, wash it with cold or lukewarm water. Do not use soap or other detergents. After a hot bath or shower, wait until your skin is completely dry and cool. Do not apply lotions, creams or ointments to the chosen area. This may prevent the transdermal patch from sticking properly.
Applying the transdermal patch:
Step 1:
Each transdermal patch is sealed in a pouch. Just before use, open the pouch by tearing it along the marked line. Take the transdermal patch.
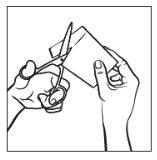
Step 2:
The adhesive side of the transdermal patch is covered by a silver protective film. Carefully peel off halfof the film. Try not to touch the adhesive part of the transdermal patch.
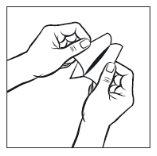
Step 3:
Stick the transdermal patch to the area of skin you have chosen and remove the rest of the film.
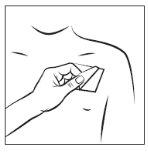
Step 4:
Press the transdermal patch against your skin with the palm of your hand and count slowly to 30. Make sure the entire transdermal patch is in contact with your skin, especially the edges.
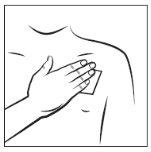
While wearing the transdermal patch
You can wear the transdermal patch for up to 4 days. If you have applied the transdermal patch correctly, the risk of it coming off is low. You can shower, bathe or swim while wearing it. However, do not expose the transdermal patch to extreme heat (e.g. sauna, infrared lamps, electric blankets or hot water bottles).
In the unlikely event that your transdermal patch falls off before you need to change it, do not use the same transdermal patch again. Apply a new one immediately (see "Changing the transdermal patch" below).
Changing the transdermal patch
? Carefully remove the old patch.
? Fold it in half with the adhesive side facing inwards.
? Dispose of it carefully, out of sight and reach of children.
? Apply a new transdermal patch to a different area of skin (as described above).
You should wait at least 1 week before applying a new patch to the same area of skin.
Duration of treatment
Your doctor will tell you how long to use Gexana. Do not stop treatment on your own, as the pain may come back and you may feel unwell (see also "If you stop using Gexana").
If you think the effect of Gexana is too strong or too weak, tell your doctor or pharmacist.
If you use more Gexana than you should
If this happens, there may be signs of a buprenorphine overdose. An overdose can intensify the adverse effects of buprenorphine, such as drowsiness, nausea and vomiting. You may have pinpoint pupils and your breathing may become slow and weak. You may also experience a cardiovascular collapse.
As soon as you realize that you have used more Gexana transdermal patches than you should, remove the excess patches and consult your doctor or pharmacist immediately.
In case of overdose or accidental ingestion, consult the Toxicology Information Service, telephone 91 562 04 20, indicating the medicine and the amount used.
If you forget to use Gexana
If you forget to apply a patch, apply a new transdermal patch as soon as you remember. This will make you change your routine, e.g. if you normally apply your transdermal patch on Mondays and Thursdays, but you forget and do not apply the new transdermal patch until Wednesday, from now on you will need to change your transdermal patches on Wednesdays and Saturdays. Note the new pair of days on the packaging calendar. If you change the patch too late, the pain may come back. In this case, consult your doctor.
Never apply more than one transdermal patch to make up for the one you forgot!
If you stop using Gexana
If you stop or finish treatment with Gexana too soon, the pain will come back. If you want to stop treatment due to unpleasant side effects, consult your doctor. Your doctor will tell you what to do and whether other medicines can be given.
Some people may have effects after using strong pain relievers for a long time and stopping them. The risk of having effects after stopping Gexana is very low. However, if you feel agitated, anxious, nervous or shaky, if you are hyperactive, have difficulty sleeping or digestive problems, consult your doctor.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Adverse effects are classified as follows:
Very Common Adverse Effects (may affect more than 1 in 10 people)
- nausea
- redness, itching
Common Adverse Effects (may affect up to 1 in 10 people)
- dizziness, headache
- shortness of breath
- vomiting, constipation
- skin changes (exanthema, usually due to repeated use), increased sweating
- edema (swelling of the legs), fatigue
Uncommon Adverse Effects (may affect up to 1 in 100 people)
- confusion, sleep disorder, restlessness
- different degrees of sedation (serenity), ranging from fatigue to confusion
- circulatory disorders (such as hypotension or rarely, loss of consciousness due to a drop in blood pressure)
- dry mouth
- rash
- urinary retention (less urine than normal), urination disorders
- fatigue
Rare Adverse Effects (may affect up to 1 in 1,000 people)
- loss of appetite
- illusions as well as hallucinations, anxiety, nightmares, decreased sexual desire
- difficulty concentrating, speech disorders, confusion, balance disorders, abnormal sensations in the skin (feeling of heat, tingling or numbness)
- vision changes, blurred vision, swollen eyelids
- hot flashes
- breathing difficulties (respiratory depression)
- stomach acid
- hives
- erection problems
- withdrawal symptoms (see below), reactions at the administration site
Very Rare Adverse Effects (may affect up to 1 in 10,000 people)
- severe allergic reactions (see below)
- dependence, mood changes
- muscle spasms, taste disorders
- pinpoint pupils
- ear pain
- abnormally rapid breathing, hiccups
- gagging
- pustules, small blisters
- chest pain
Adverse Effects with Unknown Frequency (frequency cannot be estimated from available data)
Skin disorders (usually at the application site)
- contact dermatitis (skin rash with inflammation, which may include a burning sensation), skin discoloration.
If you notice any of the above-mentioned adverse effects, consult your doctor as soon as possible.
In some cases, late local allergic reactions with marked signs of inflammation occur. In these cases, treatment with Gexana should be discontinued after consulting your doctor.
If you experience inflammation of the hands, feet, knees, face, lips, mouth, or throat, which can cause difficulty swallowing or breathing, hives, fainting, yellowing of the skin and eyes (also called jaundice), remove the transdermal patch and consult your doctor or go to the nearest hospital immediately. These may be symptoms of a very rare severe allergic reaction.
Some people may have withdrawal symptoms after using powerful painkillers for a long time and stopping their use. After treatment with Gexana, the risk of experiencing withdrawal symptoms is low. However, if you feel agitation, anxiety, nervousness, hyperactivity, sleep disorders, or digestive problems, consult your doctor.
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Conservation of Gexana
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the packaging and on the blister pack after CAD. The expiration date is the last day of the month indicated.
This medicine does not require special storage conditions.
Medicines should not be thrown away via wastewater or household waste. Deposit the packaging and medicines you no longer need at the pharmacy's SIGRE point. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Packaging and Additional Information
Composition ofGexana
The active ingredient is buprenorphine.
Each transdermal patch contains 40 mg of buprenorphine and releases approximately 70 micrograms of buprenorphine per hour.
The area of the transdermal patch containing the active ingredient is 50 cm2.
The other components are:
Adhesive matrix (containing buprenorphine):
(Z)-9-octadecen-1-yl oleate, povidone K90, 4-oxopentanoic acid, poly[acrylic acid-co-butylacrylate-co-(2-ethylhexyl)acrylate-co-vinyl acetate] (5:15:75:5), cross-linked.
Adhesive matrix(withoutbuprenorphine):
poly[acrylic acid-co-butylacrylate-co-(2-ethylhexyl)acrylate-co-vinyl acetate] (5:15:75:5), not cross-linked.
poly[acrylic acid-co-butylacrylate-co-(2-ethylhexyl)acrylate-co-vinyl acetate] (5:15:75:5), cross-linked.
Separator film between the adhesive matrices with and without buprenorphine:
poly(ethylene terephthalate) - film.
Coating layer:
polyolefin foam
Protective release liner to be removed before applying the patch: siliconized poly(ethylene terephthalate) film, coated on one side with aluminum.
Appearance of the Product and Packaging Contents
Gexana are flesh-colored transdermal patches with rounded corners, identified as: Gexana 70 μg/hour EFG, buprenorphine 40 mg.
Gexana is available in packaging containing 3, 5, 10, 20 transdermal patches individually sealed in blisters.
Only some packaging sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Aristo Pharma Iberia, S.L.
C/ Solana, 26
28850, Torrejón de Ardoz
Madrid.
Spain
Manufacturer
AMW GmbH
Birkerfeld 11
83627 Warngau
Germany
Date of the Last Revision of this Prospectus: March 2022
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to GEXANA 70 micrograms/hour transdermal patchDosage form: TRANSDERMAL PATCH, 35 micrograms/hourActive substance: buprenorphineManufacturer: Andromaco Pharma S.L.Prescription requiredDosage form: TRANSDERMAL PATCH, 52.5 micrograms/hourActive substance: buprenorphineManufacturer: Andromaco Pharma S.L.Prescription requiredDosage form: TRANSDERMAL PATCH, 70 micrograms/hourActive substance: buprenorphineManufacturer: Andromaco Pharma S.L.Prescription required
Online doctors for GEXANA 70 micrograms/hour transdermal patch
Discuss questions about GEXANA 70 micrograms/hour transdermal patch, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















