

Melodin

Ask a doctor about a prescription for Melodin

How to use Melodin
Package Leaflet: Information for the User
Melodyn, 35 micrograms/hour, transdermal system
Melodyn, 52.5 micrograms/hour, transdermal system
Melodyn, 70 micrograms/hour, transdermal system
(Buprenorphine)
You should carefully read this leaflet before using the medicine, as it contains important information for you.
- You should keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
Contents of the Package Leaflet
- 1. What is Melodyn and what is it used for
- 2. Before you use Melodyn
- 3. How to use Melodyn
- 4. Possible side effects
- 5. How to store Melodyn
- 6. Contents of the pack and other information
1. What is Melodyn and what is it used for
Melodyn is a pain-relieving medicine (analgesic) used to treat moderate to severe pain in cancer patients and severe pain in other diseases, which does not respond to non-steroidal anti-inflammatory drugs. Melodyn works through the skin. After applying the patch to the skin, the active substance buprenorphine passes through the skin into the bloodstream.
Buprenorphine belongs to a group of strong pain-relieving medicines called opioids, which reduce pain by acting on the central nervous system (on specific nerve cells in the spinal cord and brain). The effect of the patches lasts for up to three days. Melodyn is not recommended for the treatment of acute (short-term) pain.
2. Before you use Melodyn
When not to use Melodyn:
- if you are allergic to buprenorphine, soy, peanuts, or any of the other ingredients of this medicine (listed in section 6);
- if you are addicted to strong pain-relieving medicines (opioids);
- if you have a disease that causes severe breathing difficulties;
- if you are taking monoamine oxidase inhibitors (MAOIs - certain medicines used to treat depression) or if you have taken them in the last two weeks (see "Melodyn and other medicines");
- if you have myasthenia gravis (muscle weakness);
- if you have delirium tremens (a state of confusion and tremors caused by sudden withdrawal of alcohol in people who drink excessively or during an episode of excessive alcohol consumption);
- if you are pregnant.
Melodyn should not be used to treat withdrawal symptoms in people addicted to drugs.
Warnings and precautions
You should consult your doctor or pharmacist before using Melodyn in the following cases:
- after recent consumption of large amounts of alcohol;
- if you have a history of seizures or convulsions;
- if you have impaired consciousness (feeling dizzy or fainting) for unknown reasons;
- if you are in a state of withdrawal (a symptom of withdrawal may be cold sweats);
- if you have increased intracranial pressure (e.g., after head surgery or due to brain disease) and it is not possible to use artificial respiration;
- if you have breathing difficulties or are taking other medicines that can cause weakness or slowing of breathing (see "Melodyn and other medicines");
- if you have liver function disorders;
- if you tend to abuse medicines or drugs;
- if you have depression or other conditions treated with antidepressant medicines. Using these medicines at the same time as Melodyn can lead to a life-threatening condition called serotonin syndrome (see "Melodyn and other medicines").
You should also pay attention to the following warnings:
Heat and the use of external heat sources may increase the dose of buprenorphine released into the bloodstream. The use of external heat sources may cause the patch to adhere improperly to the skin. Therefore, you should not use external heat sources (sauna, infrared lamp, electric blanket, hot water bottle). You should consult your doctor if you have a fever.
Tolerance, dependence, and addiction
This medicine contains buprenorphine, which is an opioid medicine. Repeated use of opioids can lead to tolerance (the medicine becomes less effective), and long-term use can lead to dependence and addiction, which can result in life-threatening overdose. The risk of these side effects increases with higher doses and longer treatment duration.
Dependence or addiction can cause the patient to lose control over the amount of medicine taken and the frequency of its use.
The risk of dependence on Melodyn varies from person to person. The risk of dependence on Melodyn may be higher if:
- the patient or a family member has a history of drug or alcohol abuse;
- the patient is a smoker;
- the patient has a history of mood disorders (depression, anxiety, or personality disorders) or has been treated by a psychiatrist for other mental health conditions.
If any of the following symptoms occur while taking Melodyn, it may indicate dependence:
- need to take the medicine for a longer period than prescribed by the doctor
- need to take a higher dose than prescribed
- need to continue taking the medicine even if it no longer helps to relieve pain
- using the medicine for reasons other than those prescribed, such as "to feel calm" or "to fall asleep".
- repeatedly failed attempts to stop or control the use of the medicine.
- feeling unwell after stopping the medicine, and feeling better after taking it again ("withdrawal symptoms").
If any of these symptoms occur, you should talk to your doctor to discuss the best treatment option for you, including when to stop taking the medicine and how to do it safely (see also "Stopping Melodyn").
Athletes should be aware that this medicine can cause a positive reaction to doping tests.
Sleep apnea
Melodyn contains an active substance that belongs to the group of opioids. Opioids can cause sleep apnea, such as central sleep apnea (slow or interrupted breathing during sleep) and hypoxia during sleep (low oxygen levels in the blood).
The risk of central sleep apnea depends on the dose of opioids. Your doctor may consider reducing the total dose of opioids if you experience central sleep apnea.
Children and adolescents
Melodyn should not be used in people under 18 years of age, as there is no experience with the use of this medicine in this age group.
Melodyn and other medicines
You should tell your doctor or pharmacist about all medicines you are taking or have recently taken, as well as any medicines you plan to take.
Some medicines can increase the side effects of Melodyn, and sometimes cause very severe reactions. While taking Melodyn, you should not take other medicines without consulting your doctor first, especially:
- antidepressant medicines, such as moclobemide, tranylcypromine, citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, duloxetine, venlafaxine, amitriptyline, doxepin, or trimipramine. These medicines can interact with Melodyn and cause symptoms such as involuntary, rhythmic muscle contractions, including muscles that control eye movements, agitation, hallucinations, drowsiness, excessive sweating, tremors, increased reflexes, muscle tension, and body temperature above 38°C. If you experience such symptoms, you should contact your doctor.
- You should not use Melodyn at the same time as monoamine oxidase inhibitors (MAOIs - certain medicines used to treat depression) or if you have taken these medicines in the last two weeks.
- Melodyn can cause drowsiness, dizziness, or fainting, and can lead to slowed or weakened breathing. These side effects can be increased if you take other medicines that have similar side effects, such as strong pain-relieving medicines (opioids), certain sedatives, anesthetics, or medicines used to treat certain mental health conditions, such as sedatives, antidepressants, or antipsychotics.
- Taking Melodyn at the same time as sedatives, such as benzodiazepines or related medicines, increases the risk of drowsiness, breathing difficulties (respiratory depression), drowsiness, and can be life-threatening. Therefore, taking these medicines together should only be considered if other treatment options are not possible. However, if your doctor prescribes Melodyn with a sedative, the dose and duration of concomitant use should be limited. You should inform your doctor about all sedatives you are taking and follow your doctor's instructions carefully. It may be helpful to inform your friends or relatives to be aware of these symptoms. If you experience such symptoms, you should contact your doctor.
- Using Melodyn at the same time as certain medicines can increase the effect of the transdermal system. These medicines include certain antifungal or antibacterial medicines (e.g., those containing erythromycin or ketoconazole), medicines used to treat HIV infection (e.g., those containing ritonavir).
Using Melodyn at the same time as certain medicines can reduce the effect of the transdermal system. These medicines include dexamethasone; medicines used to treat epilepsy (e.g., those containing carbamazepine or phenytoin); medicines used to treat tuberculosis (e.g., rifampicin).
- Gabapentin or pregabalin used to treat epilepsy or nerve pain (neuropathic pain).
- Medicines used to treat depression.
- Medicines used to treat allergies, motion sickness, or nausea (antihistamines or antiemetics).
- Medicines used to treat mental health conditions (antipsychotics or neuroleptics).
- Muscle relaxants.
- Medicines used to treat Parkinson's disease.
Using Melodyn with food, drink, and alcohol
You should not drink alcohol while using Melodyn. Alcohol can increase certain side effects and worsen your condition.
Consuming grapefruit juice may increase the effect of Melodyn.
Pregnancy, breastfeeding, and fertility
If you are pregnant, breastfeeding, or think you may be pregnant, you should consult your doctor or pharmacist before using this medicine.
There is no sufficient experience with the use of Melodyn during pregnancy.
Therefore, you should not use Melodyn during pregnancy.
Buprenorphine, the active substance in Melodyn, inhibits milk production and passes into breast milk. Therefore, you should not use Melodyn while breastfeeding.
Driving and using machines
Melodyn can cause dizziness, drowsiness, or blurred vision, which can affect your reactions to the extent that you may not react properly or quickly enough in unexpected or emergency situations. This is especially true:
- at the start of treatment;
- when changing the dose;
- when switching from another pain-relieving medicine to Melodyn;
- if you are taking other medicines that act on the central nervous system;
- if you are drinking alcohol.
In such cases, you should not drive or operate machinery while using Melodyn. This also applies to the period after stopping Melodyn. You should not drive or operate machinery for at least 24 hours after removing the patch.
If you have any doubts or questions, you should consult your doctor or pharmacist.
Melodyn contains soybean oil. If you are allergic to peanuts or soy, you should not use this medicine.
3. How to use Melodyn
You should always use Melodyn exactly as your doctor has told you. If you are not sure, you should consult your doctor or pharmacist.
Before starting treatment and regularly during treatment, your doctor will discuss with you what you can expect from using Melodyn, when and how long to take it, when to contact your doctor, and when to stop taking it (see also "Stopping Melodyn").
Melodyn is available in three strengths: Melodyn 35 micrograms/hour, transdermal system; Melodyn 52.5 micrograms/hour, transdermal system; Melodyn 70 micrograms/hour, transdermal system.
Your doctor will decide which strength is suitable for you. If necessary, your doctor will decide to change the strength during treatment.
Usual dose:
Adults
If your doctor does not prescribe otherwise, you should apply one Melodyn patch as described below and change it at least every 3 days. To remember when to change the patch, you should write the date on the outer packaging. If your doctor prescribes additional pain-relieving medicines, you should follow your doctor's instructions carefully. Otherwise, you will not get the full benefit of using Melodyn.
Use in children and adolescents
Melodyn should not be used in people under 18 years of age due to the lack of experience with the use of this medicine in this age group.
Elderly patients
There is no need to adjust the dose in elderly patients.
Patients with kidney or liver function disorders
There is no need to adjust the dose in patients with kidney disorders or those undergoing dialysis.
Patients with liver function disorders
The effect and duration of action of Melodyn may be changed in patients with liver function disorders. Your doctor will monitor these patients more closely.
Instructions for opening a child-resistant sachet.
- 1. Cut along the markings on the sides of the sachet.
- 2. Tear the sachet open at the cut marks along the sealed edge.
- 3. Open the sachet and remove the patch.
Method of administration
Before applying the patch
- Choose a flat, hairless, clean area of skin on the upper part of the body, preferably on the chest, below the collarbone, or on the upper part of the arm. If you have difficulty applying the patch, you should ask for help.
- If the chosen area has hair, you should cut it with scissors. Do not shave!
- Avoid areas of skin that are red, irritated, or have any scars, such as large scars.
- The chosen skin area must be clean and dry. If necessary, wash the skin with cold or lukewarm water. Do not use any cleansing products. After a hot bath or shower, wait until the skin is completely dry and cool. Do not apply lotions, creams, or oils to the chosen skin area, as this may reduce the adhesion of the patch.
Applying the patch
- 1. The sachet should be opened immediately before applying the patch. Each patch is packaged in a separate sachet.
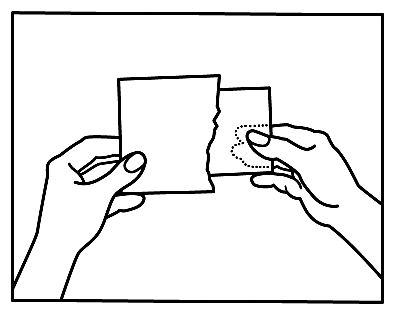
- 2. First, you should separate the loosely attached thin foil.
- 3. Then, you should remove the foil backing from half of the patch, avoiding touching the adhesive surface.
- 4. After applying half of the patch, you should remove the foil backing from the other half.
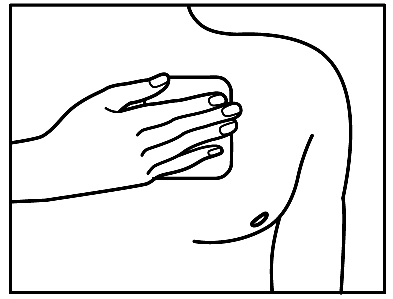
- 5. After applying the patch, you should press it firmly onto the skin with the middle part of your hand for about 30-60 seconds. You should make sure that the patch adheres well to the skin, especially at the edges.
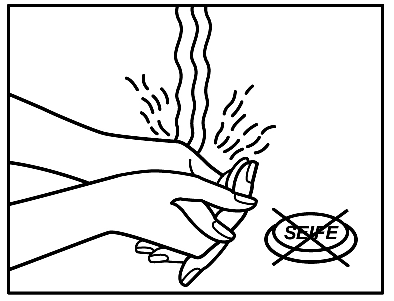
- 6. After applying the patch, you should wash your hands. Do not use any cleansing products.
After applying the patch
The patch can stay on the skin for up to 3 days. If the patch is applied correctly, there is a small risk that it will fall off. With the patch applied correctly, you can take a shower, bathe, or swim. You should not expose the patch to heat (e.g., sauna, infrared lamp, electric blanket, hot water bottle).
If the patch falls off unexpectedly before the planned change, you should not reapply it. You should apply a new patch immediately (see "Changing the patch").
Changing the patch
- Remove the old patch from the skin.
- Fold it in half, with the adhesive surfaces together.
- Dispose of the used patch with caution, so that it is not visible and not accessible to children.
- Apply a new patch to a different area of skin (following the instructions above). You can apply a new patch to the same area of skin only after one week.
Duration of treatment
Your doctor will tell you how long to use Melodyn. You should not stop using Melodyn on your own, as this may cause the pain to return and worsen your condition (see also "Stopping Melodyn").
If you feel that the effect of Melodyn is too strong or too weak, you should consult your doctor or pharmacist.
Using a higher dose of Melodyn than prescribed
If you use a higher dose of Melodyn than prescribed, you may experience symptoms of buprenorphine overdose. Overdose can lead to an increase in the side effects of buprenorphine, such as drowsiness, nausea, and vomiting. You may have pinpoint pupils, and breathing may become slow or weak. You may also experience circulatory collapse.
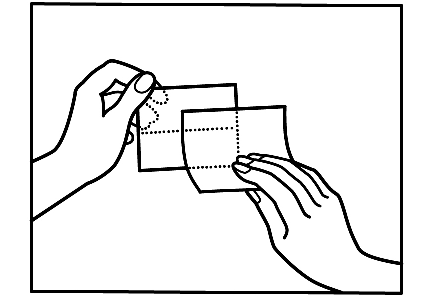
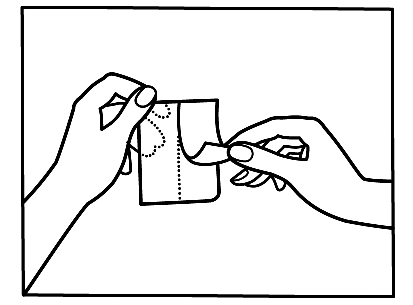
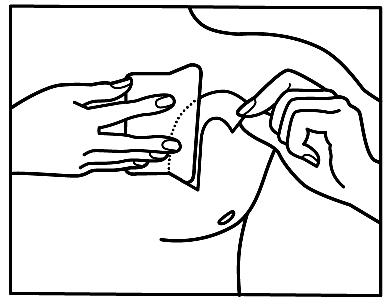
If you notice that you have applied more patches than necessary, you should remove the extra patch immediately and contact your doctor or pharmacist.
Missing a dose of Melodyn
If you forget to apply a patch, you should do so as soon as you remember. If you change the patch too late, the pain may return. In such a situation, you should contact your doctor.
You should not apply two patches to make up for a missed dose.
Stopping Melodyn
Suddenly stopping or ending the use of Melodyn may lead to the return of pain symptoms.
If you want to stop using Melodyn due to unpleasant side effects, you should first consult your doctor, who will inform you how to stop the treatment and whether you need to use other medicines.
In some patients, after long-term use of strong pain-relieving medicines, withdrawal symptoms may occur. The risk of withdrawal symptoms after stopping Melodyn is low. However, if you experience symptoms such as restlessness, anxiety, nervousness, or tremors, and you are overactive and have difficulty sleeping or digestive disorders, you should consult your doctor.
If you have any further questions or concerns about using this medicine, you should consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Melodyn can cause side effects, although not everybody gets them.
Side effects have been classified as follows:
Immune system disorders
Very rare:
severe skin reactions (see below)
Metabolism and nutrition disorders
Rare:
loss of appetite
Psychiatric disorders
Uncommon:
confusion, sleep disorders, restlessness
Rare:
hallucinations, anxiety dreams, decreased sexual desire
Very rare:
addiction, mood changes
Nervous system disorders
Common:
dizziness, headache
Uncommon:
various degrees of sedation, from fatigue to a feeling of fogginess in the head
| Very common: More than 1 in 10 people | Common: More than 1 in 100 people and less than 1 in 10 people |
| Uncommon: More than 1 in 1000 people and less than 1 in 100 people | Rare: More than 1 in 10,000 people and less than 1 in 1000 people |
| Very rare: Less than 1 in 10,000 people | Not known: cannot be estimated from the available data |
Rare:
difficulty concentrating, speech disorders, fogginess in the head, balance disorders, abnormal skin sensations (tingling, prickling, or burning of the skin)
Very rare:
muscle tremors, taste disorders
Eyes
Rare:
vision disorders, blurred vision, eyelid edema
Very rare:
pupil constriction
Ears
Very rare:
ear pain
Heart and blood vessels
Uncommon:
circulatory disorders (such as low blood pressure or, rarely, circulatory collapse)
Rare:
hot flashes
Respiratory system
Common:
slow breathing
Rare:
breathing difficulties (respiratory depression)
Very rare:
rapid breathing, hiccups
Gastrointestinal disorders
Very common:
nausea
Common:
vomiting, constipation
Uncommon:
dry mouth
Rare:
heartburn
Very rare:
vomiting reflex
Skin and subcutaneous tissue disorders (usually at the application site)
Very common:
redness, itching
Common:
skin changes (rash, usually after repeated use), sweating
Uncommon:
rash
Rare:
hives
Very rare:
pustules, small blisters
Unknown frequency:
contact dermatitis (skin rash with inflammation, which may include a burning sensation), skin discoloration
Urinary disorders
Uncommon:
urination disorders, urinary retention (less urine than usual)
Reproductive system and breast disorders
Rare:
erectile dysfunction
General disorders
Common:
edema (e.g., edema of the legs), fatigue
Uncommon:
weakness
Rare:
withdrawal symptoms (see below), reactions at the application site
Very rare:
chest pain
If you experience any of the above side effects, you should inform your doctor as soon as possible.
In some cases, delayed allergic reactions with visible symptoms of inflammation may occur. In such cases, you should stop using Melodyn after consulting your doctor.
If you experience swelling of the hands, feet, ankles, face, lips, tongue, or throat, which may cause difficulty swallowing or breathing, hives, fainting, yellowing of the skin and eyes (jaundice), you should remove the patch immediately and contact your doctor or go to the emergency department of the nearest hospital. These may be symptoms of a very rare but serious allergic reaction.
In some patients, after long-term use of strong pain-relieving medicines, withdrawal symptoms may occur. The risk of withdrawal symptoms after stopping Melodyn is low. However, if you experience symptoms such as restlessness, anxiety, nervousness, or tremors, and you are overactive and have difficulty sleeping or digestive disorders, you should inform your doctor.
If any of the side effects get worse, or if you experience any side effects not listed in this leaflet, you should inform your doctor or pharmacist.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, you should inform your doctor or pharmacist. You can also report side effects directly to the:
Department for Monitoring of Adverse Reactions to Medicinal Products, Medical Devices, and Biocides
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
You can also report side effects to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Melodyn
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the carton and pouch. The expiry date refers to the last day of that month.
Store below 25°C.
Do not freeze.
Medicines should be kept out of the reach of children. Melodyn can cause serious harm or even death if taken by someone else, either intentionally or accidentally.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
What Melodyn contains
The active substance is buprenorphine.
Melodyn 35 micrograms/hour, transdermal system: each transdermal system contains 20 mg of buprenorphine and releases 35 micrograms of buprenorphine per hour. The active surface area of the patch is 25 cm².
Melodyn 52.5 micrograms/hour, transdermal system: each transdermal system contains 30 mg of buprenorphine and releases 52.5 micrograms of buprenorphine per hour. The active surface area of the patch is 37.5 cm².
Melodyn 70 micrograms/hour, transdermal system: each transdermal system contains 40 mg of buprenorphine and releases 70 micrograms of buprenorphine per hour. The active surface area of the patch is 50 cm².
The other ingredients are:
Adhesive layer
styrene-butadiene-styrene (SBS) and styrene-butadiene block copolymers,
colophony, antioxidants (2,4-bis(1,1-dimethylpropyl)phenol (3:1), tris(2,4-ditertbutylphenyl)phosphite, aloe vera extract (also contains refined soybean oil and alpha-tocopherol acetate).
Outer layer
Polyethylene/Polyester/Aluminum, blue ink.
Protective layer (removable)
Siliconized polyester.
What Melodyn looks like and contents of the pack
Patches are flesh-colored, rectangular in shape with rounded edges, and have the following prints:
"Buprenorphin 35 µg/h".
"Buprenorphin 52,5 µg/h".
"Buprenorphin 70 µg/h".
Each transdermal system is packaged in a single heat-sealed sachet.
Melodyn is available in packs containing 4, 5, 8, 10, 16, or 24 (6x4) patches, each in a sachet, in a carton box.
Not all pack sizes may be marketed.
The following strengths are available:
Melodyn 35 micrograms/hour.
Melodyn 52.5 micrograms/hour.
Melodyn 70 micrograms/hour.
Marketing authorization holder and manufacturer
G.L. Pharma GmbH
Schlossplatz 1
8502 Lannach
Austria
For more information about this medicine, please contact the marketing authorization holder:
G.L. PHARMA POLAND Sp. z o.o.
Al. Jana Pawła II 61/313
01-031 Warsaw, Poland
Phone: 022/636 52 23; 636 53 02
[email protected]
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Germany
Buvera 35 Mikrogramm/Stunde Transdermales Pflaster
Buvera 52,5 Mikrogramm/Stunde Transdermales Pflaster
Buvera 70 Mikrogramm/Stunde Transdermales Pflaster
Czech Republic
Buprenorphine Teva 35 µg/h transdermální náplast
Buprenorphine Teva 52,5 µg/h transdermální náplast
Buprenorphine Teva 70 µg/h transdermální náplast
Poland
Melodyn 35 mikrogramów/godzinę, system transdermalny
Melodyn 52,5 mikrogramów/godzinę, system transdermalny
Melodyn 70 mikrogramów/godzinę, system transdermalny
Portugal
Buprenorfina Actavis 35 microgramas/h sistema transdermico
Buprenorfina Actavis 52,5 microgramas/h sistema transdermico
Buprenorfina Actavis 70 microgramas/h sistema transdermico
Date of last revision of the leaflet: 10.10.2024
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterActavis Group PTC ehf. G.L. Pharma GmbH Luyte Pharma AG
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MelodinDosage form: Solution, 0.3 mg/mlActive substance: buprenorphinePrescription requiredDosage form: Tablets, 0.2 mgActive substance: buprenorphinePrescription requiredDosage form: Tablets, 0.4 mgActive substance: buprenorphinePrescription required
Alternatives to Melodin in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Melodin in Spain
Alternative to Melodin in Ukraine
Online doctors for Melodin
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Melodin – subject to medical assessment and local rules.














