
BRONCORAL NEO 12 micrograms/PUFF pressurized inhalation solution

How to use BRONCORAL NEO 12 micrograms/PUFF pressurized inhalation solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: information for the user
BRONCORAL NEO 12 micrograms/puff Pressurized inhalation solution
Formoterol fumarate dihydrate
Read this leaflet carefully before starting to take this medicine, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any doubts, consult your doctor or pharmacist.
- This medicine has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the leaflet
- What is BRONCORAL NEO and what is it used for
- What you need to know before taking BRONCORAL NEO
- How to take BRONCORAL NEO
- Possible side effects
- Storage of BRONCORAL NEO
- Contents of the pack and further information
1. What is BRONCORAL NEO and what is it used for
Broncoral Neo is an inhalation medicine that allows the active ingredient to be deposited directly into your lungs. It is used to treat wheezing and breathing difficulties in patients with moderate to severe asthma. Its active ingredient, formoterol fumarate, belongs to a group of medicines called bronchodilators, which facilitate breathing by relaxing the spasms of the muscle of the airways of the lungs. Regular use of Broncoral Neo, along with corticosteroids (both inhaled and oral), will help prevent long-term respiratory problems.
Broncoral Neo may also be used to relieve symptoms such as coughing, wheezing, and difficulty breathing in patients with chronic obstructive pulmonary disease (COPD) who require regular long-term bronchodilator therapy.
2. What you need to know before taking BRONCORAL NEO
Do not useBroncoral Neoif:
- you are allergic to formoterol or any of the other components of this medicine (listed in section 6).
- to treat a sudden asthma attack.It will not help. Use a rescue inhaler for this purpose and always carry it with you.
Warnings and precautions
Consult your doctor or pharmacist before starting to take Broncoral Neo if:
- you have a serious heart disease, especially if you have recently suffered a heart attack, coronary heart disease, or severe heart muscle weakness (congestive heart failure)
- you have heart rhythm disorders, such as an increased heart rate, heart valve abnormalities, or certain electrocardiographic abnormalities, or any other heart disease
- you have narrowing of the blood vessels, especially the arteries, or abnormal enlargement of the vessel wall
- you have high blood pressure
- you have high blood sugar levels (diabetes mellitus)
- you have low potassium levels in your blood
- you have an overactive thyroid gland
- you have tumors of the adrenal cortex that produce or do not produce epinephrine
- you are going to undergo surgery or receive halogenated anesthetics.
Other medicines and Broncoral Neo
Tell your doctor or pharmacist if you are taking, have recently taken, or may need to take any other medicine.
Some medicines may interfere with the action of Broncoral Neo, such as:
- medicines for treating heart rhythm disorders (e.g., quinidine, disopyramide, procainamide)
- medicines for treating heart disease (e.g., digitalis)
- medicines for treating nasal congestion (e.g., ephedrine)
- medicines called adrenergic beta-blockers used to treat heart disease or glaucoma (increased eye pressure), whether in tablet or eye drop form
- medicines containing erythromycin (used to treat infections)
- medicines for treating depression symptoms: monoamine oxidase inhibitors (e.g., phenelzine and isocarboxazid) or tricyclic antidepressants (e.g., amitriptyline and imipramine)
- medicines for treating severe mental disorders (e.g., chlorpromazine and trifluperazine)
- medicines for treating allergic reactions (such as antihistamines, e.g., terfenadine, astemizole, mizolastine)
- medicines for treating bronchial asthma (e.g., theophylline, aminophylline, or corticosteroids)
- medicines that stimulate urine production (such as diuretics)
- medicines for treating Parkinson's disease (e.g., levodopa)
- medicines containing oxytocin, which causes uterine contractions
- medicines for treating underactive thyroid gland (e.g., thyroxine)
The addition of anticholinergics (such as tiotropium or ipratropium bromide) to the treatment with Broncoral Neo may help to further open the airways.
You have also been prescribed corticosteroids for your respiratory problems. It is very important that you continue to use them regularly. Do not stopusing them or change the dose when you start taking Broncoral Neo.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, or think you may be pregnant or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
The use of Broncoral Neo during pregnancy is only indicated if it is absolutely necessary.
Driving and using machines
It is unlikely that Broncoral Neo will have any influence on your ability to drive or use machines.
Broncoral Neo contains ethanol
This medicine contains small amounts of ethanol (alcohol): 8.9 mg of alcohol (ethanol) per puff, which corresponds to 0.25 mg/kg per dose of two puffs in adults and equivalent to 0.44 mg/kg per dose of two puffs in adolescents. The amount in two puffs of this medicine is equivalent to less than 1 ml of wine or beer. The small amount of alcohol in this medicine has no noticeable effects.
3. How to take BRONCORAL NEO
Follow the instructions for taking this medicine exactly as indicated by your doctor. If in doubt, consult your doctor.
Asthma
The dose of Broncoral Neo that you should inhale will depend on the type and severity of the asthma you suffer from. Your dose will be determined by your doctor, and it is essential that you take only the prescribed dose, regularly.
The usual dose for adults, including the elderly and adolescents from 12 years old, is 1 puff in the morning and 1 puff in the evening. This means that you need to take a total daily dose of Broncoral Neo of 2 puffs (24 micrograms).
If you suffer from more severe asthma, your doctor may prescribe 4 puffs per day (48 micrograms), 2 puffs in the morning and 2 puffs in the evening.
The maximum daily doseof Broncoral Neo is 48 micrograms (4 puffs), and it is essential that you do not takemorethan the total daily dose prescribed by your doctor.
This productis not suitable for asthmatic children under 12 years old.
Chronic Obstructive Pulmonary Disease (COPD)
The usual dose for adults (over 18 years old), including the elderly, is 1 puff in the morning and 1 puff in the evening. This means that you need to take a total of 2 puffs (24 micrograms) of Broncoral Neo per day.
If you suffer from more severe COPD, your doctor may prescribe 4 puffs per day (48 micrograms), 2 puffs in the morning and 2 puffs in the evening.
The maximum daily doseof Broncoral Neo is 48 micrograms (4 puffs), and it is essential that you do not takemorethan the total daily dose prescribed by your doctor.
Do not take more than 2 puffs at the same time.
This product is not suitable for patients with COPD under 18 years old.
Do not use Broncoral Neo to treat an acute asthma attack.For this, you should use a "rescue" inhaler prescribed by your doctor, which you should always carry with you.
If you take moreBroncoral Neothan you should
You may notice an increased heart rate and have tremors. You may also experience headache, tremors, nausea, and vomiting, or feel drowsy. Inform your doctor as soon as possible.
If youforget to use Broncoral Neo
If you forget to take Broncoral Neo, take it as soon as you remember. If it is almost time for your next dose, do not take the missed dose and continue with your usual dosing schedule. Do not take a double dose to make up for missed doses.
Do not stop taking or reducethe dose of Broncoral Neo or any other medicine for your respiratory disease simply because you feel better, without consulting your doctor first. It is very important to use these medicines regularly.
Do not increaseyour dose of Broncoral Neo without consulting your doctor first.
If you experience difficulty breathing or wheezing while using Broncoral Neo, you should continue using Broncoral Neo but should visit your doctor as soon as possible; you may need additional treatment. Once your asthma is well controlled, your doctor may consider it appropriate to gradually reduce the dose of Broncoral Neo.
Instructions for use
It is essential that you know how to use your inhaler correctly. Your doctor, nurse, or pharmacist will teach you how to use your inhaler correctly. You must carefully follow their instructions to learn how, when, and how manypuffs you should administer. This leaflet details the correct instructions. If you are unsure about how to proceed or have difficulties with inhalation, consult your doctor, nurse, or pharmacist.
Checking your inhaler.If your inhaler is new or has not been used for 3 days or more, you must discharge a puff into the air to ensure it is working correctly.
Whenever possible, you should be standing or sitting upright when applying the inhalation.
1. Remove the protective cap from the mouthpiece of the inhaler and hold it between your thumb and index finger as shown.
2. Exhale as deeply as possible.
3. Hold the canister vertically, as shown, with your thumb on the base of the mouthpiece; place the inhaler in your mouth, between your teeth, and close your lips firmly.
4. Inhale deeply through your mouth and, at the same time, press the top of the inhaler to release a puff.
5. Hold your breath for as long as possible without straining and finally remove the inhaler from your mouth.
- If you need another inhalation, keep the inhaler in a vertical position and wait for half a minute, then repeat steps 2 to 5.
- After use, replace the protective cap to prevent it from getting dirty. Put it on firmly and leave it in place.
1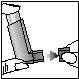 2
2 3
3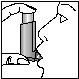 4
4 5-6
5-6 7
7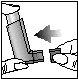
IMPORTANT. Do not perform steps 2, 3, 4, and 5 too quickly.
Before inhalation, it is essential that you breathe as slowly as possible.
If some of the gas escapes from the top of the inhaler or from the corner of your lips, Broncoral Neo will not reach your lungs as it should. Take another puff carefully, following the instructions from step 2.
If you have weakness in your hands, you may find it easier to hold the inhaler with both hands. Place your two index fingers on the top of the inhaler and your two thumbs on the bottom of the inhaler.
If you have any difficulties, consult your doctor, nurse, or pharmacist.
Cleaning the inhaler
It is essential to clean the inhaler regularly, at least once or twice a week, to ensure proper functioning.
- Remove the aluminum canister from the plastic inhaler and remove the protective cap from the mouthpiece.
- Rinse the plastic inhaler and the protective cap with warm water.
- Do not get the aluminum canister wet with water.
- Dry the inhaler and the protective cap well in a warm place. Avoid high temperatures.
- Replace the aluminum canister and the protective cap of the mouthpiece.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The following are the possible side effects according to their frequency. If you are unsure about the side effects mentioned, consult your doctor to explain them to you.
If you experience worsening of breathing difficulties or wheezing after using the inhaler, stop using Broncoral Neo andinform your doctor immediately. This is caused by a narrowing of the airways in your lungs, although it occurs only rarely.
Common(affecting at least 1 in 10 people):
- exceptionally rapid heartbeats and palpitations, coughing, tremors (mild tremor), headache.
Uncommon(affecting at least 1 in 100 people):
- muscle cramps, muscle pain, nausea, agitation, nervousness, sleep disorders, dizziness, taste disturbances, throat irritation, increased heart rate, heart rhythm disorders with increased heart rate, decreased potassium levels in the blood, increased blood sugar levels, increased insulin levels, free fatty acids, glycerol, and ketones, excessive sweating.
Rare(affecting at least 1 in 1,000 people):
- absence of heartbeats caused by premature contraction of the ventricles, feeling of chest pressure, increased or decreased blood pressure, spasm of the bronchial muscles after using inhaled medicines, severe decrease in blood pressure, kidney inflammation, hypersensitivity reactions such as itching, skin rash, bronchospasm, hives, swelling of the skin and mucous membranes persisting for several days.
Very rare(affecting at least 1 in 10,000 people):
- acute worsening of asthma, difficulty breathing, swelling of the hands and/or feet, irregular heartbeats, reduction in the number of platelets, hyperexcitability, abnormal behavior, hallucinations.
Some side effects, such as tremors, nausea, taste disturbances, throat irritation, excessive sweating, nervousness, headache, dizziness, and muscle cramps, may resolve spontaneously after one to two weeks of continued treatment.
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the Spanish Medicines Monitoring System for Human Use https://www.notificaram.es.
By reporting side effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of BRONCORAL NEO
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after three months of purchase from the pharmacy, or after the expiration date shown on the box and label. The expiration date is the last day of the month indicated.
- Do not store above 30°C.
- If the inhaler is very cold, remove the aluminum canister from the inhaler and warm it by holding it in your handsfor a few minutes before use. Neveruse any other heat source.
- Caution. The aluminum canister contains a pressurized liquid. Do not expose to temperatures above 50°C. Also, do not puncture the container.
- Medicines should not be disposed of via wastewater or household waste. Place the containers and medicines you no longer need in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the containers and medicines you no longer need. This will help protect the environment.
6. Container Content and Additional Information
Broncoral Neo Composition
- The active ingredient of Broncoral Neo is formoterol fumarate dihydrate. Each puff provides 12 micrograms of formoterol fumarate dihydrate. This corresponds to a released dose of 10.1 micrograms.
- The other components are: hydrochloric acid, ethanol, and norflurane (HFC-134a).
This medication contains fluorinated greenhouse gases.
Each 50-puff inhaler contains 5.842 g of norflurane (HFC-134a), which corresponds to 0.008 tons of CO2 equivalent (global warming potential GWP = 1430).
Each 100-puff inhaler contains 9.087 g of norflurane (HFC-134a), which corresponds to 0.013 tons of CO2 equivalent (global warming potential GWP = 1430).
Each 120-puff inhaler contains 10.384 g of norflurane (HFC-134a), which corresponds to 0.015 tons of CO2 equivalent (global warming potential GWP = 1430).
Product Appearance and Container Content
Broncoral Neo is presented as an inhalation solution in a pressurized aluminum container, equipped with a plastic inhaler and protective cap.
Each container contains 1 inhaler that provides 50, 100, or 120 puffs.
Only some package sizes may be marketed.
Marketing Authorization Holder
Chiesi España, S.A.U.
Plaça d’Europa, 41-43, Planta 10
08908 L’Hospitalet de Llobregat
Barcelona (Spain)
Manufacturer and Batch Release Responsible
Chiesi Farmaceutici S.p.A., Vía San Leonardo 96, 43122 Parma, Italy
Alternative Manufacturer and Batch Release Responsible
Chiesi Pharmaceuticals GmbH, Gonzagagasse 16/16, 1010 Wien, Austria
This medication has been authorized in the following Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following trademarks:
Germany | ATIFOR CHIESI | Hungary | ATIMOS |
Estonia | ATIMOS | Portugal | ATIMOS |
Spain | BRONCORAL NEO | Slovenia | ATIMOS |
Netherlands | ATIMOS | Poland | ATIMOS |
United Kingdom (Northern Ireland) | ATIMOS MODULITE | Latvia | ATIMOS |
Czech Republic | ATIMOS | ||
Slovak Republic | ATIMOS | ||
Date of the Last Revision of this Prospectus:July 2024.
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es.
- Country of registration
- Average pharmacy price37.81 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BRONCORAL NEO 12 micrograms/PUFF pressurized inhalation solutionDosage form: PULMONARY INHALATION, 12 mcg formoterol fumarateActive substance: formoterolManufacturer: Bexal Farmaceutica S.A.Prescription requiredDosage form: PULMONARY INHALATION, 12 microgramsActive substance: formoterolManufacturer: Viatris Healthcare LimitedPrescription requiredDosage form: PULMONARY INHALATION, 12 MCGActive substance: formoterolManufacturer: Laboratorio Aldo Union S.L.Prescription required
Online doctors for BRONCORAL NEO 12 micrograms/PUFF pressurized inhalation solution
Discuss questions about BRONCORAL NEO 12 micrograms/PUFF pressurized inhalation solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















