
FENILEFRINE ALTAN 0.1 mg/mL INJECTABLE SOLUTION AND PERFUSION SOLUTION

How to use FENILEFRINE ALTAN 0.1 mg/mL INJECTABLE SOLUTION AND PERFUSION SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Phenylephrine Altan 0.1 mg/ml Solution for Injection and Infusion
Phenylephrine
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again. - If you have any further questions, ask your doctor, pharmacist, or nurse.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Phenylephrine Altan 0.1 mg/ml and what is it used for
- What you need to know before you use Phenylephrine Altan 0.1 mg/ml
- How to use Phenylephrine Altan 0.1 mg/ml
- Possible side effects
- Storage of Phenylephrine Altan 0.1 mg/ml
- Contents of the pack and further information
1. What is Phenylephrine Altan 0.1 mg/ml and what is it used for
This medicine belongs to a group called adrenergic or dopaminergic agents.
Phenylephrine Altan 0.1 mg/ml is used to treat low blood pressure (hypotension) that may occur during different types of anesthesia.
2. What you need to know before you use Phenylephrine Altan 0.1 mg/ml
Do not use Phenylephrine Altan 0.1 mg/ml
- If you are allergic (hypersensitive) to phenylephrine or any of the other ingredients of this medicine (listed in section 6).
- If you are being treated with a medicine known as a monoamine oxidase inhibitor or have stopped treatment with this type of medicine in the last 14 days.
- If you have high blood pressure.
- If you have peripheral vascular disease (poor blood circulation).
- If you have excessive thyroid gland activity.
Warnings and precautions
Caution is required when administering Phenylephrine Altan 0.08 mg/ml to patients with:
- pre-existing cardiovascular disease
- diabetes mellitus
- arterial hypertension
- ischemic heart disease
- arrhythmia
- bradycardia
- incomplete cardiac block
- tachycardia
- peripheral vascular occlusive disease, including arteriosclerosis
- aneurysm
- in patients with angina pectoris, phenylephrine may precipitate or exacerbate angina.
- angle-closure glaucoma.
Phenylephrine may induce a reduction in cardiac output. Therefore, caution is required when administering to patients with atherosclerosis, the elderly, and patients with compromised cerebral or coronary circulation.
In patients with severe cardiac failure or cardiogenic shock, phenylephrine may worsen cardiac failure as a consequence of induced vasoconstriction and increased afterload.
Special attention should be paid to the injection of phenylephrine to prevent extravasation, as this can cause tissue necrosis.
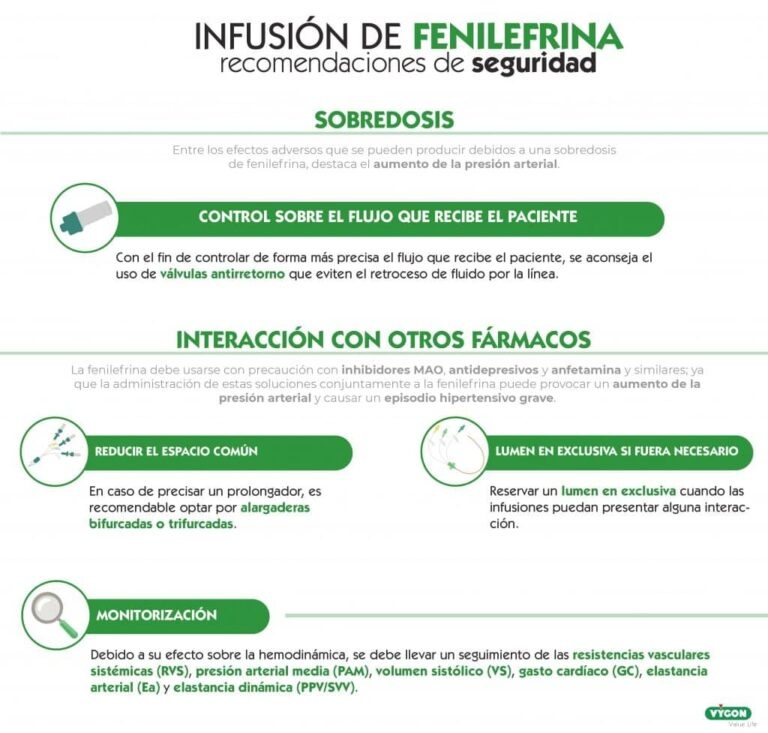
Using Phenylephrine Altan 0.1 mg/ml with other medicines
Tell your doctor or nurse if you are using, have recently used, or might use any other medicines.
Phenylephrine Altan 0.1 mg/ml may interact with the following medicines:
Contraindicated combinations (see section 4.3)
Non-selective monoamine oxidase inhibitors (MAOIs) (phenelzine, tranylcypromine)
Paroxysmal hypertension, possibly fatal hyperthermia. Due to the long duration of MAOI inhibitory action, this interaction is still possible 15 days after the MAOI inhibitor is stopped.
Not recommended combinations
Dopaminergic alkaloids (bromocriptine, cabergoline, lisuride, pergolide):
Risk of vasoconstriction and/or hypertensive crisis.
Vasoconstrictor alkaloids (dihydroergotamine, ergotamine, methylergonovine, methysergide):
Risk of vasoconstriction and/or hypertensive crisis.
Tricyclic antidepressants (e.g., imipramine):
Paroxysmal hypertension with possible arrhythmia (inhibition of adrenaline or noradrenaline entry into sympathetic fibers).
Noradrenergic-serotonergic antidepressants (milnacipran, venlafaxine):
Paroxysmal hypertension with possible arrhythmia (inhibition of adrenaline or noradrenaline entry into sympathetic fibers).
Selective monoamine oxidase inhibitors type A (moclobemide, toloxatone)
Risk of vasoconstriction and/or hypertensive episodes.
Linezolid:
Risk of vasoconstriction and/or hypertensive crisis.
Guanethidine and related products:
Substantial increase in blood pressure (hyperreactivity related to reduced sympathetic tone and/or inhibition of adrenaline or noradrenaline entry into sympathetic fibers). If combination cannot be avoided, use with caution lower doses of sympathomimetic agents.
Cardiac glycosides, quinidine:
Increased risk of arrhythmias.
Sibutramine
Paroxysmal hypertension with possible arrhythmias (inhibition of adrenaline or noradrenaline entry into sympathetic fibers).
Halogenated volatile anesthetics (desflurane, enflurane, halothane, isoflurane, methoxyflurane, sevoflurane):
Risk of hypertensive crises and perioperative arrhythmia.
Combinations that require precautions for use:
Antihypertensives, including alpha and beta receptor blockers.
Phenylephrine may increase blood pressure and thus counteract the action of many antihypertensive agents. The interaction between phenylephrine and alpha and beta receptor blockers may be complex. Medicines that act on alpha-1 adrenoreceptors may potentiate the action of phenylephrine (such as granisetron) or decrease it (such as doxazosin or buspirone).
Oxytocic agents:
The effect of presoactive sympathomimetic amines is potentiated. Therefore, some oxytocic agents may cause severe persistent hypertension and cerebrovascular accidents may occur during the postpartum period.
Children
The use of this medicine is not recommended in children due to insufficient data on efficacy, safety, and dosage recommendations.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor, pharmacist, or nurse before taking this medicine.
Pregnancy
When administered in the late stages of pregnancy or during labor, Phenylephrine Altan 0.1 mg/ml may harm the fetus. Phenylephrine injection may be used during pregnancy according to the indications.
Breastfeeding
The amount of phenylephrine hydrochloride that passes into breast milk appears to be small.
Driving and using machines
There is no information on how Phenylephrine Altan 0.1 mg/ml affects your ability to drive and use machines.
Phenylephrine Altan 0.1 mg/ml contains sodium
This medicine contains 366.2 mg of sodium (main component of table/cooking salt) in each 100 ml. This is equivalent to 18.3% of the maximum recommended daily sodium intake for an adult.
3. How to use Phenylephrine Altan 0.1 mg/ml
Follow exactly the administration instructions of this medicine as indicated by your doctor. In case of doubt, consult your doctor or pharmacist again.
Phenylephrine Altan 0.1 mg/ml should always be administered by a healthcare professional and never by the patient (see section 6).
The recommended dose is:
- Intravenous bolus injection:
The normal dose is 50 to 100 micrograms, which may be repeated until the desired effect is achieved. A bolus dose should not exceed 100 micrograms.
- Continuous infusion:
The initial dose is 25 to 50 micrograms/min. The doses may be increased or decreased to maintain systolic blood pressure near the normal value. Doses between 25 and 100 micrograms/min have been found to be effective.
Pediatric population
The safety and efficacy of phenylephrine in children under 18 years have not been established. No data are available.
Renal impairment
Lower doses of phenylephrine may be required in patients with renal impairment.
Hepatic impairment
Higher doses of phenylephrine may be required in patients with hepatic cirrhosis.
Advanced age
Treatment in elderly patients should be carried out with caution.
If you use more Phenylephrine Altan 0.1 mg/ml than you should
Symptoms of overdose include headache, nausea, vomiting, paranoid psychosis, hallucinations, increased blood pressure, reflex bradycardia, and irregular heart rhythm. In case of overdose or accidental ingestion, consult your doctor, pharmacist, or nurse immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
Treatment should consist of symptomatic and supportive measures. The hypertensive effects (high blood pressure) may be treated with medicines known as alpha-adrenergic blockers, such as phentolamine, 5-60 mg intravenously over 10-30 minutes, repeated as necessary.
If you forget to use Phenylephrine Altan 0.1 mg/ml
Do not use a double dose to make up for a forgotten dose. If you have any doubts about the use of this medicine, consult your doctor or pharmacist.
Phenylephrine Altan 0.1 mg/ml contains sodium
This medicine contains 366.2 mg of sodium (main component of table/cooking salt) in each 100 ml. This is equivalent to 18.3% of the maximum recommended daily sodium intake for an adult.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Side effects can be classified according to their frequency as follows: Very common (may affect more than 1 in 10 people), Common (may affect up to 1 in 10 people), Uncommon (may affect up to 1 in 100 people), Rare (may affect up to 1 in 1,000 people), Very rare (may affect up to 1 in 10,000 people), and Not known (frequency cannot be estimated from the available data)
The most common side effects of phenylephrine are bradycardia, hypertensive episodes, nausea, and vomiting. Hypertension is more frequent with high doses.
The most commonly reported cardiovascular side effect appears to be bradycardia, probably due to vagal stimulation mediated by baroreceptors and consistent with the pharmacological effect of phenylephrine.
The following adverse reactions have been reported during the use of phenylephrine, although their frequency has not been clearly established:
Immune system disorders:
hypersensitivity
Psychiatric disorders:
anxiety, excitability, agitation, psychotic states, confusion
Nervous system disorders:
headache, cerebral hemorrhage, vertigo, fainting, lethargy (mental or physical inactivity), insomnia, paresthesia (abnormal skin sensation), tremor (involuntary shaking of the body or limbs)
Eye disorders:
mydriasis, worsening of pre-existing angle-closure glaucoma
Cardiac disorders:
reflex bradycardia (slow heart rate), reflex tachycardia (fast heart rate), cardiac arrhythmia (irregular heart rhythm), angina pectoris, palpitations, cardiac arrest
Vascular disorders:
hypertension (high blood pressure), hypotension (low blood pressure), flushing (redness), hypertensive crisis
Respiratory, thoracic, and mediastinal disorders:
dyspnea (breathing difficulties), pulmonary edema (inflammation of the lung)
Gastrointestinal disorders:
nausea, vomiting, hypersalivation
Skin and subcutaneous tissue disorders:
sweating, temporary tingling, skin cooling, pallor or skin paleness, piloerection
Renal and urinary disorders:
difficulty urinating, urinary retention
Metabolism and nutrition disorders:
alterations in glucose metabolism
General disorders and administration site conditions:
extravasation of phenylephrine may cause tissue necrosis (tissue death)
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes possible side effects not listed in this leaflet.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report them directly through the Spanish Medicines Surveillance System for Human Use: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Phenylephrine Altan 0.1 mg/ml
Keep this medicine out of the sight and reach of children.
This medicine does not require any special storage temperature for polypropylene bags.
Do not store above 30°C for polyolefin bags without PVC.
Store in the original packaging to protect from light.
Do not use this medicine after the expiry date that appears on the packaging after EXP. The expiry date is the last day of the month indicated.
Do not freeze
Your doctor will store this medicine for you.
From a microbiological point of view, the product should be used immediately after the first opening.
Phenylephrine Altan 0.1 mg/ml should not be used if visible particles are present in it.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and further information
Composition of Phenylephrine Altan 0.1 mg/ml
- The active ingredient is phenylephrine hydrochloride. Each bag contains 10 mg of phenylephrine hydrochloride, equivalent to 8 mg of phenylephrine base.
- The other ingredients are sodium chloride, sodium citrate, citric acid, and water for injections.
Appearance and packaging of the product
Phenylephrine Altan 0.1 mg/ml is a clear and colorless solution. Each polypropylene or polyolefin PVC-free bag contains 100 ml of solution.
Package sizes
Polypropylene bag: 10 x 100 ml
Polyolefin PVC-free bag: 10 x 100 ml
Marketing authorization holder and manufacturer
Marketing authorization holder
Altan Pharmaceuticals S.A.
C/ Cólquide, Nº 6, Portal 2, 1ª Planta, Oficina F.
Edificio Prisma, Las Rozas, 28230 Madrid – Spain
Manufacturer
Altan Pharmaceuticals, S.A.
Polígono Industrial de Bernedo, s/n,
01118 Bernedo, Álava.- Spain
Date of the last revision of this leaflet:January 2021
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
----------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Phenylephrine Altan 0.1 mg/ml solution for injection and infusion
Posology and method of administration:
Parenteral administration or intravenous infusion. Phenylephrine Altan 0.1 mg/ml solution for injection and infusion should only be administered by healthcare professionals with adequate training and experience.
1. Intravenous bolus injection:
The normal dose is 50 to 100 micrograms, which may be repeated until the desired effect is achieved. A bolus dose should not exceed 100 micrograms.
- Continuous infusion:
The initial dose is 25 to 50 micrograms/min. The doses may be increased or decreased to maintain systolic blood pressure near the normal value. Doses between 25 and 100 micrograms/min have been found to be effective.
Elderly patients:
Treatment of elderly patients should be carried out with caution.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FENILEFRINE ALTAN 0.1 mg/mL INJECTABLE SOLUTION AND PERFUSION SOLUTIONDosage form: INJECTABLE, 100 micrograms/mlActive substance: phenylephrineManufacturer: Laboratoire AguettantPrescription requiredDosage form: INJECTABLE, 50 micrograms/mlActive substance: phenylephrineManufacturer: Laboratoire AguettantPrescription requiredDosage form: INJECTABLE, 10 mg/mLActive substance: phenylephrineManufacturer: Altan Pharmaceuticals SaPrescription required
Online doctors for FENILEFRINE ALTAN 0.1 mg/mL INJECTABLE SOLUTION AND PERFUSION SOLUTION
Discuss questions about FENILEFRINE ALTAN 0.1 mg/mL INJECTABLE SOLUTION AND PERFUSION SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











