EYLEA 40 mg/ml INJECTABLE SOLUTION IN VIAL

How to use EYLEA 40 mg/ml INJECTABLE SOLUTION IN VIAL
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Eylea 40 mg/ml solution for injection in vial
aflibercept
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor.
- If you get any side effects, talk to your doctor, even if you think they might be related to the injection. See section 4.
Contents of the pack
- What is Eylea and what is it used for
- What you need to know before you are given Eylea
- How Eylea will be given
- Possible side effects
- Storing Eylea
- Contents of the pack and other information
1. What is Eylea and what is it used for
Eylea is a solution that is injected into the eye to treat certain eye diseases in adult patients, known as:
- neovascular (exudative) age-related macular degeneration, commonly known as wet AMD
- vision impairment due to macular edema following branch or central retinal vein occlusion (BRVO or CRVO)
- vision impairment due to diabetic macular edema (DME)
- vision impairment due to myopic choroidal neovascularization (myopic CNV).
Aflibercept, the active substance in Eylea, blocks the activity of a group of factors known as vascular endothelial growth factor A (VEGF-A) and placental growth factor (PlGF).
In patients with wet AMD and myopic CNV, when these factors are present in excess, they promote the formation of abnormal new blood vessels in the eye. These new blood vessels can cause leakage of blood components into the eye, resulting in damage to the tissues responsible for vision.
In patients with CRVO, there is a blockage of the main vein that carries blood from the retina. As a result, VEGF levels increase, causing fluid leakage in the retina and subsequent swelling of the macula (the part of the retina responsible for fine vision), known as macular edema.
When the macula becomes filled with fluid, central vision becomes blurred.
In patients with BRVO, there is a blockage of one or more branches of the main blood vessel that carries blood from the retina. As a result, VEGF levels increase, causing fluid leakage in the retina and subsequent swelling of the macula.
Diabetic macular edema is a swelling of the retina that occurs in patients with diabetes due to fluid leakage from the blood vessels in the macula. The macula is the part of the retina responsible for fine vision. When the macula becomes swollen with fluid, central vision becomes blurred.
Eylea has been shown to stop the growth of abnormal new blood vessels in the eye that often bleed or leak fluid. Eylea may help to stabilize and, in many cases, improve vision loss caused by wet AMD, CRVO, BRVO, DME, and myopic CNV.
2. What you need to know before you are given Eylea
You should not be given Eylea
- if you are allergic to aflibercept or any of the other ingredients of this medicine (listed in section 6)
- if you have an active infection or suspect that you may have an infection in or around the eye (ocular or periocular infection)
- if you have severe inflammation of the eye (indicated by pain or redness).
Warnings and precautions
Tell your doctor before you are given Eylea:
- If you have glaucoma.
- If you have a history of flashes of light or floaters or if you suddenly experience an increase in the size and number of floaters.
- If you have had or are scheduled to have eye surgery within the previous 4 weeks or the next 4 weeks.
- If you have a severe form of CRVO or BRVO (ischemic CRVO or BRVO), treatment with Eylea is not recommended.
Additionally, it is important that you know that:
- The safety and efficacy of Eylea when administered in both eyes at the same time have not been studied, and if used in this way, it may increase the risk of side effects.
- Eylea injections may cause an increase in pressure inside the eye (intraocular pressure) in some patients within 60 minutes after the injection. Your doctor will monitor you after each injection.
- If you develop an infection or inflammation inside the eye (endophthalmitis) or other complications, you may notice pain or increased discomfort in the eye, worsening of redness, blurred or decreased vision, and increased sensitivity to light. It is essential that any symptoms that appear are diagnosed and treated as soon as possible.
- Your doctor will check if you have other risk factors that may increase the likelihood of a tear or detachment of the layers at the back of the eye (retinal tear or detachment, or retinal pigment epithelial tear or detachment), in which case Eylea will be administered with caution.
- Eylea should not be used during pregnancy, unless the potential benefit outweighs the potential risk to the fetus.
- Women of childbearing age must use effective contraceptive methods during treatment and for at least three months after the last injection of Eylea.
The systemic use of VEGF inhibitors, substances similar to those contained in Eylea, is potentially associated with the risk of blood clots blocking blood vessels (arterial thromboembolic events) that can lead to a heart attack or stroke. After injection of Eylea into the eye, there is a theoretical risk that these events may occur. Data on the safety of treatment in patients with CRVO, BRVO, DME, and myopic CNV who have had a stroke, transient ischemic attack, or myocardial infarction in the last 6 months are limited. If any of these conditions apply to you, Eylea will be administered with caution.
Experience is limited in the treatment of:
- Patients with DME due to type 1 diabetes.
- Diabetic patients with very high average blood sugar levels (HbA1c above 12%).
- Diabetic patients with a severe eye disease caused by diabetes, known as proliferative diabetic retinopathy.
There is no experience in the treatment of:
- Patients with acute infections.
- Patients with other eye diseases such as retinal detachment or macular hole.
- Diabetic patients with uncontrolled hypertension.
- Non-Asian patients with myopic CNV.
- Patients who have been previously treated for myopic CNV.
- Patients with damage outside the central part of the macula (extrafoveal lesions) due to myopic CNV.
If any of the above applies to you, your doctor will take into account this lack of information when treating you with Eylea.
Children and adolescents
Eylea has not been studied in children and adolescents under 18 years of age, as wet AMD, CRVO, BRVO, DME, and myopic CNV mainly occur in adults. Therefore, its use in this age group is not recommended.
Using Eylea with other medicines
Tell your doctor if you are using, have recently used, or might use any other medicines.
Pregnancy and breastfeeding
- Women of childbearing age must use effective contraceptive methods during treatment and for at least three months after the last injection of Eylea.
- There is no experience with the use of Eylea in pregnant women. Eylea should not be used during pregnancy, unless the potential benefit outweighs the potential risk to the fetus. If you are pregnant or planning to become pregnant, tell your doctor before treatment with Eylea.
- Small amounts of Eylea may pass into breast milk. The effects on newborns/infants are unknown. Eylea is not recommended during breastfeeding. If you are breastfeeding, tell your doctor before treatment with Eylea.
Driving and using machines
After injection of Eylea, you may experience temporary visual disturbances. Do not drive or use machines while these disturbances last.
Eylea contains
- less than 1 mmol of sodium (23 mg) per dose; this is essentially "sodium-free".
- 0.015 mg of polysorbate 20 in each 0.05 ml dose, equivalent to 0.3 mg/ml. Polysorbates may cause allergic reactions. Tell your doctor if you have any known allergies.
3. How Eylea will be given
Eylea will be given to you by a doctor who is experienced in giving eye injections, under aseptic conditions (clean and sterile).
The recommended dose is 2 mg of aflibercept (0.05 ml).
Eylea is given as an injection into the eye (intravitreal injection).
Before the injection, your doctor will use an antiseptic eye wash to carefully clean your eye to prevent infection. Your doctor will also give you a local anesthetic to reduce or prevent any pain you might feel with the injection.
Wet AMD
Patients with wet AMD will be treated with one injection per month for the first three doses, followed by another injection after two months.
Your doctor will then decide if the treatment interval between injections can be maintained every two months or gradually extended to 2 or 4 weeks if your disease has stabilized. If your disease worsens, the interval between injections may be shortened.
You do not need to visit your doctor between injections, unless your doctor thinks it is necessary or you experience any problems.
Macular edema secondary to branch or central retinal vein occlusion
Your doctor will determine the most suitable treatment schedule for you. Your treatment will start with a series of Eylea injections given once a month.
The interval between two injections should not be less than one month.
Your doctor may decide to stop treatment with Eylea if you do not benefit from continued treatment.
Treatment will continue with one injection per month until your disease has stabilized. You may need three or more monthly injections.
Your doctor will monitor your response to treatment and may continue treatment, gradually increasing the interval between injections to stabilize your disease. If your disease worsens with a longer treatment interval, your doctor will shorten the interval between injections.
Based on your response to treatment, your doctor will decide on the follow-up schedule.
Diabetic macular edema (DME)
Patients with DME will be treated with one injection per month for the first five consecutive doses, and then one injection every two months.
The interval between treatments may be maintained every two months or adjusted according to your disease, based on the examination by your doctor. Your doctor will decide on the follow-up schedule.
Your doctor may decide to stop treatment with Eylea if you do not benefit from continued treatment.
Myopic choroidal neovascularization (myopic CNV)
Patients with myopic CNV will be treated with a single injection. You will only receive further injections if your doctor's examinations reveal that your disease has not improved.
The interval between two injections should not be less than one month.
If your disease disappears and then returns, your doctor may restart treatment.
Your doctor will decide on the follow-up schedule.
If you miss an injection of Eylea
Make a new appointment to have the injection.
Stopping treatment with Eylea
Talk to your doctor before stopping treatment.
If you have any further questions on the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
It is possible that allergic reactions(hypersensitivity) may occur. These can be serious andrequire you to contact your doctor immediately.
With the administration of Eylea, some side effects may occur that affect the eyes and are due to the injection procedure. Some of these can be serious, including blindness, a severe infectionor inflammation inside the eye(endophthalmitis), detachment, tear, or hemorrhage of the light-sensitive layer at the back of the eye(retinal detachment, retinal tear), clouding of the lens(cataract), bleeding in the eye(vitreous hemorrhage), detachment of the gel-like substance inside the eye from the retina(vitreous detachment), and increased pressure inside the eye(see section 2). These serious eye side effects occurred in less than 1 in 1,900 injections during clinical trials.
If you notice a sudden decrease in vision or an increase in pain and redness in the eye after the injection, contact your doctor immediately.
List of reported side effects
The following is a list of side effects that have been reported as possibly related to the injection procedure or the medicine. Do not be alarmed, as you may not experience any of them. Always talk to your doctor about any suspected side effect.
Very common side effects(may affect more than 1 in 10 people):
- visual impairment
- bleeding in the back of the eye (retinal hemorrhage)
- blood in the eye due to bleeding from small blood vessels in the outer layers of the eye
- eye pain
Common side effects(may affect up to 1 in 10 people):
- detachment or tear of one of the layers at the back of the eye that produce flashes of light with floaters that may progress to vision loss (retinal pigment epithelial tear or detachment, retinal tear or detachment)
- retinal degeneration (causing visual disturbances)
- bleeding in the eye (vitreous hemorrhage)
- certain types of clouding of the lens (cataract)
- damage to the outer layer of the eyeball (cornea)
- increased pressure inside the eye
- floaters in the vision
- detachment of the gel-like substance inside the eye from the retina (vitreous detachment, resulting in flashes of light with floaters)
- sensation of having something in the eye
- increased tear production
- swelling of the eyelid
- bleeding at the injection site
- redness of the eye
- Side effects that are known to be associated with wet AMD; observed only in patients with wet AMD.
Uncommon side effects(may affect up to 1 in 100 people):
- allergic reactions (hypersensitivity)**
- severe infection or inflammation inside the eye (endophthalmitis)
- inflammation of the iris or other parts of the eye (iritis, uveitis, iridocyclitis, cells in the anterior chamber)
- abnormal sensation in the eye
- irritation of the eyelid
- swelling of the outer layer of the eyeball (cornea)
- Allergic reactions were reported as rash, itching (pruritus), hives (urticaria), and some cases of serious allergic reactions (anaphylactic/anaphylactoid reactions).
Rare side effects(may affect up to 1 in 1,000 people):
- blindness
- clouding of the lens due to injury (traumatic cataract)
- inflammation of the gel-like substance inside the eye
- pus in the eye
Frequency not known(cannot be estimated from the available data):
- inflammation of the white part of the eye associated with redness and pain (scleritis)
In clinical trials, an increase in the incidence of bleeding from small blood vessels in the outer layers of the eye (conjunctival hemorrhage) was observed in patients with wet AMD who were treated with anticoagulant medicines. This increase in incidence was comparable in patients treated with ranibizumab and Eylea.
The systemic use of VEGF inhibitors, substances similar to those contained in Eylea, is potentially associated with the risk of blood clots blocking blood vessels (arterial thromboembolic events) that can lead to a heart attack or stroke. After injection of Eylea into the eye, there is a theoretical risk that these events may occur. Data on the safety of treatment in patients with CRVO, BRVO, DME, and myopic CNV who have had a stroke, transient ischemic attack, or myocardial infarction in the last 6 months are limited. If any of these conditions apply to you, Eylea will be administered with caution.
As with all therapeutic proteins, there is a possibility of an immune reaction (formation of antibodies) with Eylea.
Reporting of side effects
If you experience any side effects, talk to your doctor, even if you think they might be related to the injection. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Eylea
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the carton and on the label after “EXP”. The expiry date refers to the last day of the month.
- Store in a refrigerator (between 2 °C and 8 °C). Do not freeze.
- The unopened vial can be stored outside the refrigerator below 25 °C for a maximum of 24 hours.
- Store in the original package to protect from light.
- Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Package contents and additional information
Composition of Eylea
- The active substance is: aflibercept. One vial contains an extractable volume of at least 0.1 ml, equivalent to at least 4 mg of aflibercept. One vial provides a dose of 2 mg of aflibercept in 0.05 ml.
- The other ingredients are: polysorbate 20 (E 432), sodium dihydrogen phosphate monohydrate (for pH adjustment), disodium hydrogen phosphate heptahydrate (for pH adjustment), sodium chloride, sucrose, water for injections.
See “Eylea contains” in section 2 for more information.
Appearance and package contents of the product
Eylea is an injectable solution (injectable) in a vial. The solution is colorless to pale yellow.
Package with 1 vial + 1 filter needle.
Marketing authorization holder
Bayer AG
51368 Leverkusen
Germany
Manufacturer
Bayer AG
Müllerstraße 178
13353 Berlin
Germany
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
België / Belgique / Belgien Bayer SA-NV Tel: +32-(0)2-535 63 11 | Lietuva UAB Bayer Tel: +370-5-233 68 68 |
България Байер България ЕООД Тел: +359-(0)2-424 72 80 | Luxembourg / Luxemburg Bayer SA-NV Tel: +32-(0)2-535 63 11 |
Ceská republika Bayer s.r.o. Tel: +420-266 101 111 | Magyarország Bayer Hungária KFT Tel: +36-1-487 4100 |
Danmark Bayer A/S Tlf: +45-45 235 000 | Malta Alfred Gera and Sons Ltd. Tel: +356-21 44 62 05 |
Deutschland Bayer Vital GmbH Tel: +49-(0)214-30 513 48 | Nederland Bayer B.V. Tel: +31–23-799 1000 |
Eesti Bayer OÜ Tel: +372-655 85 65 | Norge Bayer AS Tlf: +47-23 13 05 00 |
Ελλάδα Bayer Ελλάς ΑΒΕΕ Τηλ: +30-210-618 75 00 | Österreich Bayer Austria Ges. m. b. H. Tel: +43-(0)1-711 460 |
España Bayer Hispania S.L. Tel: +34-93-495 65 00 | Polska Bayer Sp. z o.o. Tel: +48-22-572 35 00 |
France Bayer HealthCare Tél (N° vert): +33-(0)800 87 54 54 | Portugal Bayer Portugal, Lda. Tel: +351-21-416 42 00 |
Hrvatska Bayer d.o.o. Tel: + 385-(0)1-6599 900 | România SC Bayer SRL Tel: +40-(0)21-529 59 00 |
Ireland Bayer Limited Tel: +353-(0)1-216 3300 | Slovenija Bayer d. o. o. Tel: +386-(0)1-58 14 400 |
Ísland Icepharma hf. Sími: +354-540 80 00 | Slovenská republika Bayer, spol. s r.o. Tel: +421-(0)2-59 21 31 11 |
Italia Bayer S.p.A. Tel: +39-02-3978 1 | Suomi/Finland Bayer Oy Puh/Tel: +358-(0)20-78521 |
Κύπρος NOVAGEM Limited Τηλ: +357-22-48 38 58 | Sverige Bayer AB Tel: +46-(0)8-580 223 00 |
Latvija SIA Bayer Tel: +371-67 84 55 63 |
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: https://www.ema.europa.eu.
If you want local information, scan here to access the website https://www.pi.bayer.com/eylea2.
A QR code with the link to the leaflet is included.
<------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
The vial must be used for the treatment of a single eye.
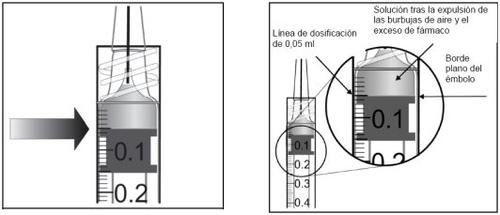
The vial contains more than the recommended dose of 2 mg of aflibercept (equivalent to 0.05 ml). The excess volume must be discarded before administration.
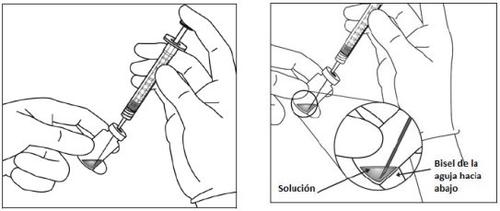
Before administration, the solution must be visually inspected for the presence of particles and/or a change in color or any change in physical appearance. If any of these are observed, do not use the medicine.
The unopened vial of Eylea can be stored outside the refrigerator below 25°C for a maximum of 24 hours. After opening the vial, proceed under aseptic conditions.
For intravitreal injection, a 30 G x ½ inch (1.27 cm) injection needle must be used.
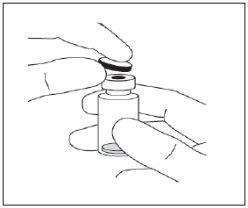
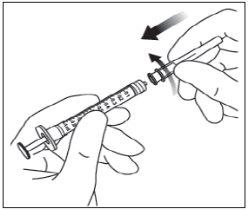
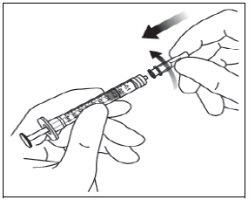
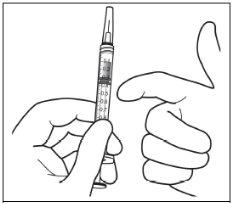
Instructions for use of the vial:
|
|
|
|
| |
| |
| |
| |
Note: The filter needle must not be used for intravitreal injection. | |
|
|
|
|
| |
| |
The disposal of unused medicine and all materials that have come into contact with it will be carried out in accordance with local regulations. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to EYLEA 40 mg/ml INJECTABLE SOLUTION IN VIALDosage form: INJECTABLE, 40 mg/mlActive substance: afliberceptManufacturer: Sandoz GmbhPrescription requiredDosage form: INJECTABLE, 40 mg/mlActive substance: afliberceptManufacturer: Sandoz GmbhPrescription requiredDosage form: INJECTABLE, 40 mg/mlActive substance: afliberceptManufacturer: Celltrion Healthcare Hungary Kft.Prescription required
Online doctors for EYLEA 40 mg/ml INJECTABLE SOLUTION IN VIAL
Discuss questions about EYLEA 40 mg/ml INJECTABLE SOLUTION IN VIAL, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions