
EXOCIN 3 mg/ml EYE DROPS SOLUTION

How to use EXOCIN 3 mg/ml EYE DROPS SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
EXOCIN 3 mg/ml Eye Drops Solution
Ofloxacin
Read the entire package leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- This medication has been prescribed to you only and should not be given to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What Exocin is and what it is used for
- What you need to know before using Exocin
- How to use Exocin
- Possible side effects
- Storage of Exocin
- Contents of the pack and further information
1. What Exocin is and what it is used for
Exocin belongs to the group of medications known as antibacterial agents of the 4-quinolone type.
Antibiotics are used to treat bacterial infections and are not effective against viral infections.
It is essential to follow the instructions regarding dosage, administration interval, and treatment duration indicated by your doctor.
Do not store or reuse this medication. If you have any leftover antibiotic after completing treatment, return it to the pharmacy for proper disposal. Do not throw medications down the drain or in the trash.
Exocin is an eye drop solution used to treat external eye infections, such as conjunctivitis and keratoconjunctivitis, in adults and children over one year of age, caused by organisms sensitive to this medication. It is also used to treat bacterial corneal ulcers in adults and children.
2. What you need to know before using Exocin
Do not use Exocin if:
You are allergic (hypersensitive) to ofloxacin, other quinolones, or any of the other components of this medication (listed in section 6).
Warnings and precautions:
This product should be used with caution in patients with an allergy (hypersensitivity) to other antibacterial agents of the quinolone type. If a skin rash or any other sign of an allergic reaction appears during treatment, discontinue treatment and consult your doctor.
This product should be used with caution in patients with an anomaly or ulceration of the eye surface. If this is the case, consult your doctor. Prolonged use of ofloxacin may cause a new bacterial infection in the eye, which may not be effectively treated with Exocin.
Exocin may increase sensitivity to sunlight. Avoid direct exposure to sunlight while using Exocin.
Heart problems
You should be cautious when using this type of medication if you have been born with or have a family history of prolonged QT interval (detected on an ECG, heart electrical recording), if you have an electrolyte imbalance in the blood (mainly low potassium or magnesium levels), if you have a very slow heart rate (called "bradycardia"), if you have a weakened heart (heart failure), if you have a history of heart attacks (myocardial infarction), if you are a woman or an elderly patient, or if you are using other medications that cause abnormal ECG changes (see section "Using other medications").
Children and adolescents:
The use of Exocin is not recommended in children under one year of age. It is not recommended for use in newborns.
Other medications and Exocin
Inform your doctor or pharmacist if you are using or have recently used other medications, including those purchased without a prescription.
You should inform your doctor if you are using other medications that may affect heart rhythm: medications that belong to the group of antiarrhythmics (such as quinidine, hydroquinidine, disopyramide, amiodarone, sotalol, dofetilide, ibutilide), tricyclic antidepressants, some antibiotics (those belonging to the macrolide group), and some antipsychotics.
Pregnancy and breastfeeding
Consult your doctor or pharmacist before using any medication.
The use of Exocin is not recommended during pregnancy and breastfeeding. If necessary, your doctor will assess the convenience or not of its use.
Driving and using machines
There are no studies on the effect of Exocin on the ability to drive and use machines. Due to the possibility of causing blurred vision, you should wait until it has disappeared before driving or using machines.
Exocin contains benzalkonium chloride
This medication contains 0.05 mg of benzalkonium chloride per ml of solution.
Benzalkonium chloride can be absorbed by soft contact lenses, altering their color. Remove contact lenses before using this medication and wait 15 minutes before putting them back.
Benzalkonium chloride can cause eye irritation, especially if you have dry eye or other corneal diseases (the transparent layer on the front of the eye). Consult your doctor if you feel any strange sensation, itching, or pain in the eye after using this medication.
3. How to use Exocin
Follow your doctor's instructions for using Exocin exactly. Consult your doctor or pharmacist if you have any questions.
Exocin is administered via the eye. The normal dose is:
For the treatment of external eye infections in adults and children over one year of age: Apply one or two drops to the affected eye(s) every two to four hours during the first two days and then four times a day.
For the treatment of bacterial corneal ulcers in adults and children: During the first two days, apply one or two drops every 30 minutes while awake. At night, apply one or two drops at four and six hours after going to bed. From day 3 to day 7 or 9, apply one or two drops every hour while awake. From day 7 or 9 onwards, apply one or two drops four times a day.
Instructions for use
Do not use the product if the seal on the neck of the container is broken before opening it for the first time.
Apply the eye drops as follows:
1. 2.
2. 3.
3. 4.
4.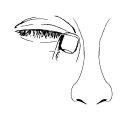
- Wash your hands before opening the container. Tilt your head back and look up.
- Gently pull down the lower eyelid until a small pocket is formed.
- Squeeze the inverted container to release one or two drops into each eye.
- While keeping the eye closed, press your finger against the corner of your eye (near your nose) and hold it for 30 seconds.
- Repeat steps 2, 3, and 4 for the other eye if it also needs treatment.
If a drop falls outside the eye, repeat the operation.
To avoid contaminating the eye, do not let the dropper tip touch the eye or any other surface. Put the container upright, replace the cap, and tighten it to close the container. Clean any excess liquid from your cheek with a clean cloth.
After opening the container (breaking the seal), do not use the eye drops for more than 28 days.
If you have any questions, consult your doctor or pharmacist.
Use in children
There is very limited information on the use of Exocin in children.
If your doctor has prescribed Exocin for the treatment of your child and you want to obtain more information about this medication, please consult your doctor again. If your child becomes ill during treatment with Exocin, please contact your doctor immediately.
If you use more Exocin than you should
If you have put too many drops in the eye(s), rinse your eyes with fresh water. Then, apply the eye drops following the usual routine.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medication and the amount ingested.
If you forget to use Exocin
If you forget to use Exocin, apply it as soon as you remember, unless it is almost time for the next dose, in which case skip the missed dose. Apply your next dose and continue with your usual routine. Do not use a double dose to make up for missed doses.
If you stop using Exocin
Do not stop using Exocin or reduce the amount you are using until your doctor tells you to. Otherwise, your symptoms may worsen.
If you have any other questions about using this product, ask your doctor or pharmacist.
Treatment duration
Your doctor will indicate the duration of your treatment with Exocin. Do not stop treatment before, as the medication may not have the desired effect.
Treatment of external eye infections should not exceed 10 days, unless your doctor indicates otherwise.
4. Possible side effects
Like all medications, Exocin can have side effects, although not everyone experiences them. The following side effects have been reported, but their exact frequency may vary.
There have been reports of skin rash (Stevens-Johnson syndrome, toxic epidermal necrolysis) with the use of Exocin, which can be potentially fatal, initially appearing as red spots or circular patches, often with central blisters on the trunk.
Frequent (may affect up to 1 in 10 people):
Affecting the eye: Eye irritation; eye discomfort
Frequency not known (cannot be estimated from available data):
Affecting the eye: Blurred vision, sensitivity to light, eye redness, itching, allergic reaction in the eye (including itching in the eye and eyelid), foreign body sensation in the eye, increased tearing, inflammation around the eyes (including eyelid inflammation), dry eye, eye pain.
Affecting the body: Dizziness, nausea, allergic reactions (including swelling under the skin that can occur in areas such as the face, lips, or other parts of the body; rash, itching, or hives; swelling of the mouth, tongue, or throat that can obstruct the airways and can cause wheezing, difficulty swallowing, breathing, or shortness of breath; a sudden, severe, and life-threatening allergic reaction), facial swelling.
Affecting the heart: Abnormally fast heart rate, life-threatening irregular heart rhythm, alteration of heart rhythm (called "prolongation of the QT interval", detected on an ECG, heart electrical recording)
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. You can also report them directly through the Spanish Medicines and Health Products Agency's website: https://www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Exocin
Keep this medication out of sight and reach of children. Do not use this medication after the expiration date stated on the label and carton, after "EXP". The expiration date is the last day of the month indicated.
Do not store above 25°C.
Do not use Exocin if you notice a change in color or if the contents become cloudy.
Discard the medication 28 days after opening,even if there is still some solution left. To help you remember, write the opening date on the space provided on the carton.
Medications should not be thrown down the drain or in the trash. Deposit the containers and medications you no longer need in the pharmacy's SIGRE collection point. If you have any doubts, ask your pharmacist how to dispose of the containers and medications you no longer need. This will help protect the environment.
6. Contents of the pack and further information
Composition of Exocin
- The active ingredient is ofloxacin 3 mg/ml
- The other ingredients are: benzalkonium chloride, sodium chloride, sodium hydroxide (to adjust pH), hydrochloric acid (to adjust pH), and purified water.
Appearance of Exocin and contents of the pack
Exocin is a solution in a plastic bottle with a dropper and screw cap. Each bottle contains 5 ml of solution.
Marketing authorization holder and manufacturer
Marketing authorization holder:
AbbVie Spain, S.L.U.
Avenida de Burgos 91,
28050 Madrid
Spain
Manufacturer:
Allergan Pharmaceuticals Ireland
Castlebar Road
Westport
County Mayo
Ireland
This package leaflet was approved in November 2020
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.es.
- Country of registration
- Average pharmacy price2.83 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to EXOCIN 3 mg/ml EYE DROPS SOLUTIONDosage form: EYEDROP, 3 mg/mlActive substance: ofloxacinManufacturer: Brill Pharma S.L.Prescription requiredDosage form: EYEDROP, 5 mg/mlActive substance: moxifloxacinManufacturer: Tiedra Farmaceutica S.L.Prescription requiredDosage form: EYEDROP, 3mg/mlActive substance: ciprofloxacinManufacturer: Laboratorios Salvat S.A.Prescription required
Online doctors for EXOCIN 3 mg/ml EYE DROPS SOLUTION
Discuss questions about EXOCIN 3 mg/ml EYE DROPS SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















