Enurev Breezhaler 44 micrograms powder for inhalation

How to use Enurev Breezhaler 44 micrograms powder for inhalation
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Enurev Breezhaler 44 micrograms inhalation powder (hard capsule)
glycopyrronium
(as glycopyrronium bromide)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Enurev Breezhaler and what is it used for
- What you need to know before you use Enurev Breezhaler
- How to use Enurev Breezhaler
- Possible side effects
- Storing Enurev Breezhaler
- Contents of the pack and other information
1. What is Enurev Breezhaler and what is it used for
What is Enurev Breezhaler
This medicine contains the active substance glycopyrronium bromide, which belongs to a class of medicines called bronchodilators.
What Enurev Breezhaler is used for
This medicine is used to make breathing easier for adult patients who have difficulty breathing due to a lung disease called chronic obstructive pulmonary disease (COPD).
In COPD, the muscles around the airways contract, making it difficult to breathe. This medicine blocks the contraction of these muscles in the lungs, making it easier for air to enter and leave the lungs.
If you use this medicine once a day, it will help reduce the effects of COPD on your daily life.
2. What you need to know before you use Enurev Breezhaler
Do not use Enurev Breezhaler
- if you are allergic to glycopyrronium bromide or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor before you start using Enurev Breezhaler, if you have:
- kidney problems.
- a known eye condition called narrow-angle glaucoma.
- difficulty urinating.
During treatment with Enurev Breezhaler,stop using this medicine and tell your doctor immediately:
- if you notice chest tightness, coughing, wheezing, or difficulty breathing immediately after using Enurev Breezhaler (signs of bronchospasm).
- if you have difficulty breathing or swallowing, swelling of the tongue, lips, or face, rash, itching, and hives (signs of an allergic reaction).
- if you notice pain or discomfort in the eyes, blurred vision, halos, or colored images in association with eye redness. These may be signs of an acute attack of narrow-angle glaucoma.
Enurev Breezhaler is used as a maintenance treatment for your COPD. Do not use this medicine to treat a sudden attack of shortness of breath or wheezing.
Children and adolescents
Do not give this medicine to children or adolescents under 18 years of age.
Other medicines and Enurev Breezhaler
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines. These include medicines similar to Enurev Breezhaler used for your lung disease, such as ipratropium, oxitropium, or tiotropium (also known as anticholinergics).
No specific side effects have been reported when Enurev Breezhaler is used with other medicines used to treat COPD, such as rescue inhalers (e.g., salbutamol), methylxanthines (e.g., theophylline), and/or oral and inhaled steroids (e.g., prednisolone).
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
No data are available on the use of this medicine in pregnant women, and it is not known whether the active substance of this medicine passes into breast milk.
Driving and using machines
This medicine is unlikely to affect your ability to drive or use machines.
Enurev Breezhaler contains lactose
This medicine contains lactose. If your doctor has told you that you have an intolerance to some sugars, contact them before taking this medicine.
3. How to use Enurev Breezhaler
Follow exactly the instructions for using this medicine given by your doctor or pharmacist. If you are unsure, ask your doctor or pharmacist again.
How much Enurev Breezhaler to use
The usual dose is to inhale the contents of one capsule each day.
You only need to inhale the medicine once a day since the effect of this medicine lasts 24 hours.
Do not use more than the dose prescribed by your doctor.
Elderly
If you are 75 years of age or older, you can use this medicine at the same dose as other adults.
When to inhale Enurev Breezhaler
Use this medicine at the same time each day. This will help you remember to use it.
You can inhale this medicine at any time before or after food or drink.
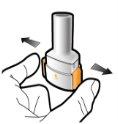
How to inhale Enurev Breezhaler
- In this pack, you will find an inhaler and capsules (in blisters) that contain the medicine in powder form for inhalation. Use the capsules only with the inhaler provided in this pack (Enurev Breezhaler inhaler). The capsules should be kept in the blister until you need to use them.
- Do not push the capsule through the foil.
- When starting a new pack, use the new Enurev Breezhaler inhaler provided in the pack.
- Discard the inhaler from each pack once you have used all the capsules.
- Do not swallow the capsules.
- For more information on how to use the inhaler, please read the instructions at the end of this leaflet.
If you use more Enurev Breezhaler than you should
If you have inhaled too much of this medicine or if someone uses your capsules accidentally, tell your doctor immediately or go to the nearest emergency room. Show the pack of Enurev Breezhaler. Medical attention may be needed.
If you forget to use Enurev Breezhaler
If you miss a dose, inhale it as soon as possible. However, do not inhale two doses on the same day. Then inhale the next dose at the usual time.
How long to continue treatment with Enurev Breezhaler
- Continue using your treatment with this medicine for as long as your doctor tells you.
- COPD is a long-term disease, and you should use this medicine every day and not just when you have breathing problems or other symptoms of COPD.
Ask your doctor or pharmacist if you have any questions about how long to continue treatment with this medicine.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some side effects may be serious but are rare
(may affect up to 1 in 100 people)
- Irregular heartbeat
- High blood sugar levels (hyperglycemia: typical symptoms include excessive thirst or hunger and frequent urination)
- Rash, itching, hives, difficulty breathing or swallowing, dizziness (possible signs of an allergic reaction)
- Swelling, mainly of the tongue, lips, face, or throat (possible signs of angioedema)
If you experience any of these side effects, tell your doctor immediately.
Some side effects may be serious but the frequency is not known
(cannot be estimated from the available data)
- Difficulty breathing with wheezing or coughing (signs of paradoxical bronchospasm)
Some side effects are common
(may affect up to 1 in 10 people)
- Dry mouth
- Difficulty sleeping
- Runny or stuffy nose, sneezing, sore throat
- Diarrhea or abdominal pain
- Musculoskeletal pain
Some side effects are uncommon
(may affect up to 1 in 100 people)
- Difficulty or pain when urinating
- Painful or frequent urination
- Palpitations
- Rash
- Numbness
- Coughing with phlegm
- Tooth decay
- Pain or pressure in the cheeks and forehead
- Nosebleeds
- Pain in arms or legs
- Pain in the muscles, bones, or joints of the chest
- Stomach upset after meals
- Sore throat
- Tiredness
- Weakness
- Itching
- Voice changes (hoarseness)
- Nausea
- Vomiting
Some patients over 75 years of age experienced headache (commonly) and urinary tract infection (commonly).
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Enurev Breezhaler
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and blister after ‘EXP’. The expiry date is the last day of the month stated.
Do not store above 25°C.
Store the capsules in the original blister to protect them from moisture. Remove them from the blister just before use.
The inhaler from each pack should be discarded once you have used all the capsules.
Do not use this medicine if you notice the pack is damaged or shows signs of deterioration.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Enurev Breezhaler
- The active ingredient is glycopyrronium bromide. Each capsule contains 63 micrograms of glycopyrronium bromide (equivalent to 50 micrograms of glycopyrronium). The delivered dose (the dose released by the inhaler mouthpiece) is equivalent to 44 micrograms of glycopyrronium.
- The other ingredients of the powder for inhalation are lactose monohydrate and magnesium stearate.
Appearance of Enurev Breezhaler and Package Contents
The hard capsules of Enurev Breezhaler 44 micrograms powder for inhalation are transparent and orange in color and contain a white powder. They have the product code «GPL50» printed in black above a black line and the company logo () printed in black below it.
Each package contains a device known as an inhaler, along with capsules in blisters. Each blister strip contains 6 or 10 hard capsules.
The following package sizes are available:
Packages containing 6x1, 10 x 1, 12 x 1 or 30 x 1 hard capsules, along with an inhaler.
Multiple package containing 90 (3 packages of 30 x 1) hard capsules and 3 inhalers.
Multiple package containing 96 (4 packages of 24 x 1) hard capsules and 4 inhalers.
Multiple package containing 150 (15 packages of 10 x 1) hard capsules and 15 inhalers.
Multiple package containing 150 (25 packages of 6 x 1) hard capsules and 25 inhalers.
Only some package sizes may be marketed.
Marketing Authorization Holder
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublin 4
Ireland
Manufacturer
Novartis Farmacéutica SA
Gran Via de les Corts Catalanes, 764
08013 Barcelona
Spain
Novartis Pharma GmbH
Roonstraße 25
D-90429 Nuremberg
Germany
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Belgium Novartis Pharma N.V. Tel: +32 2 246 16 11 | Lithuania SIA Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
| Luxembourg Novartis Pharma N.V. Tel: +32 2 246 16 11 |
Czech Republic Novartis s.r.o. Tel: +420 225 775 111 | Hungary Novartis Hungária Kft. Tel.: +36 1 457 65 00 |
Denmark Novartis Healthcare A/S Tlf: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Germany Novartis Pharma GmbH Tel: +49 911 273 0 | Netherlands Novartis Pharma B.V. Tel: +31 88 04 52 111 |
Estonia SIA Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Norway Novartis Norge AS Tlf: +47 23 05 20 00 |
Greece Novartis (Hellas) A.E.B.E. Tel: +30 210 281 17 12 | Austria Novartis Pharma GmbH Tel: +43 1 86 6570 |
Spain Ferrer Internacional, S.A. Tel: +34 93 600 37 00 | Poland Novartis Poland Sp. z o.o. Tel.: +48 22 375 4888 |
France Novartis Pharma S.A.S. Tel: +33 1 55 47 66 00 | Portugal Novartis Farma - Produtos Farmacêuticos, S.A. Tel: +351 21 000 8600 |
Croatia Novartis Hrvatska d.o.o. Tel. +385 1 6274 220 | Romania Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Ireland Novartis Ireland Limited Tel: +353 1 260 12 55 | Slovenia Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Iceland Vistor hf. Tel: +354 535 7000 | Slovakia Novartis Slovakia s.r.o. Tel: +421 2 5542 5439 |
Italy Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Finland Novartis Finland Oy Tel: +358 (0)10 6133 200 |
Cyprus Novartis Pharma Services Inc. Tel: +357 22 690 690 | Sweden Novartis Sverige AB Tel: +46 8 732 32 00 |
Latvia SIA Novartis Baltics Tel: +371 67 887 070 | United Kingdom (Northern Ireland) Novartis Ireland Limited Tel: +44 1276 698370 |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu
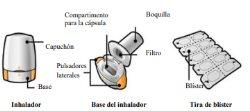
Instructions for Use of Enurev Breezhaler Inhaler
Read the Instructions for Usecompletely before using Enurev Breezhaler
Insert |
Puncture and Release |
Inhale Deeply |
Check that the Capsule is Empty |
|
|
|
|
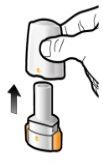
Step 1a: Remove the Cap
Step 1b: Open the Inhaler
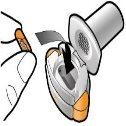
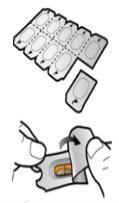
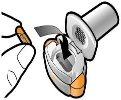
Step 1c: Remove the Capsule Separate one of the blisters from the blister strip. Open the blister and remove a capsule. Do not push the capsule through the foil. Do not swallow the capsule. |
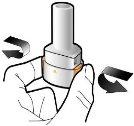
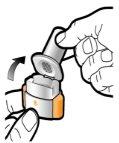
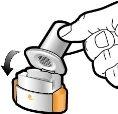
Step 2a: Puncture the Capsule Once Hold the inhaler in a vertical position. Puncture the capsule by pressing both buttons firmly at the same time. You should hear a noise when the capsule is punctured. Puncture the capsule only once.
Step 2b: Release the Buttons Completely |
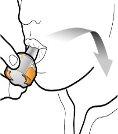
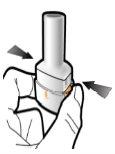
Step 3a: Exhale Completely Do not blow into the inhaler.
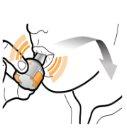
Step 3b: Inhale the Medication Deeply Hold the inhaler as shown in the figure. Insert the mouthpiece into your mouth and close your lips firmly around it. Do not press the buttons. Inhale quickly and as deeply as possible. During inhalation, you will hear a humming noise. You may notice the taste of the medication when you inhale.
Step 3c: Hold Your Breath Hold your breath for 5 seconds. |
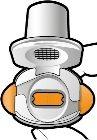
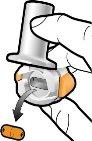
Check that the Capsule is Empty Open the inhaler to check if there is any powder left in the capsule. If there is powder left in the capsule: Close the inhaler. Repeat steps 3a to 3c.
Remove the empty capsule Discard the empty capsule in your household trash. Close the inhaler and put the cap back on. |
Step 1d: Insert the Capsule Never place the capsule directly into the mouthpiece.
Step 1e: Close the Inhaler | Important Information
|
Your Enurev Breezhaler package contains:
| Frequently Asked Questions Why did the inhaler not make a noise when I inhaled? The capsule may be stuck in the compartment. If this happens, carefully release the capsule by tapping the base of the inhaler. Inhale the medication again, repeating steps 3a to 3c. What should I do if there is powder left in the capsule? You have not received enough of your medication. Close the inhaler and repeat steps 3a to 3c. I coughed after inhaling, is it important? It may happen. If the capsule is empty, it means you have received enough of your medication. I notice small fragments of the capsule on my tongue, is it important? It may happen. It is not harmful. The likelihood of the capsules breaking into fragments increases if the capsule is punctured more than once. | Cleaning the Inhaler Wipe the mouthpiece from the inside and outside with a clean, dry cloth that does not leave lint to remove any powder residue. Keep the inhaler dry. Never wash your inhaler with water. |
Disposal of the Inhaler after Use Each inhaler should be discarded after all the capsules have been used. Ask your pharmacist how to dispose of medicines and inhalers that are no longer needed. |
- Country of registration
- Average pharmacy price47.61 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Enurev Breezhaler 44 micrograms powder for inhalationDosage form: PULMONARY INHALATION, 50 microgramsActive substance: glycopyrronium bromideManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: PULMONARY INHALATION, 50 microgramsActive substance: glycopyrronium bromideManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: PULMONARY INHALATION, 0.0040 g/aerosolActive substance: ipratropium bromideManufacturer: Laboratorio Aldo Union S.L.Prescription required
Online doctors for Enurev Breezhaler 44 micrograms powder for inhalation
Discuss questions about Enurev Breezhaler 44 micrograms powder for inhalation, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions