Tovanor Breezhaler 44 micrograms powder for inhalation

How to use Tovanor Breezhaler 44 micrograms powder for inhalation
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Tovanor Breezhaler44microgramsinhalation powder (hard capsule)
glycopyrronium
(as glycopyrronium bromide)
Read the entire package leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor, pharmacist, or nurse.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are side effects not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Tovanor Breezhaler and what is it used for
- What you need to know before taking Tovanor Breezhaler
- How to use Tovanor Breezhaler
- Possible side effects
- Storage of Tovanor Breezhaler
- Package contents and additional information
1. What is Tovanor Breezhaler and what is it used for
What is Tovanor Breezhaler
This medication contains an active ingredient called glycopyrronium bromide, which belongs to a class of medications called bronchodilators.
What is Tovanor Breezhaler used for
This medication is used to facilitate breathing in adult patients who have difficulty breathing due to a lung disease called chronic obstructive pulmonary disease (COPD).
In COPD, the muscles surrounding the airways contract, making breathing difficult. This medication blocks the contraction of these muscles in the lungs, making it easier for air to enter and leave the lungs.
If you use this medication once a day, it will help reduce the effects of COPD on your daily life.
2. What you need to know before taking Tovanor Breezhaler
Do not use Tovanor Breezhaler
- if you are allergic to glycopyrronium bromide or any of the other ingredients of this medication (listed in section 6).
Warnings and precautions
Consult your doctor before starting to use Tovanor Breezhaler if you have any of the following conditions:
- if you have kidney problems.
- if you have a known eye disorder called narrow-angle glaucoma.
- if you have difficulty urinating.
During treatment with Tovanor Breezhaler,stop taking this medication and inform your doctor immediately:
- if you notice chest tightness, coughing, wheezing, or difficulty breathing immediately after using Tovanor Breezhaler (signs of bronchospasm).
- if you have difficulty breathing or swallowing, swelling of the tongue, lips, or face, skin rash, itching, and hives (signs of an allergic reaction).
- if you notice pain or discomfort in the eyes, blurred vision, halos, or colored images in association with eye redness. These may be signs of an acute attack of narrow-angle glaucoma.
Tovanor Breezhaler is used as a maintenance treatment for your COPD. Do not use this medication to treat a sudden attack of shortness of breath or wheezing.
Children and adolescents
Do not give this medication to children or adolescents under 18 years of age.
Other medications and Tovanor Breezhaler
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medications. These include medications similar to Tovanor Breezhaler used for your lung disease, such as ipratropium, oxitropium, or tiotropium (also known as anticholinergics).
No specific side effects have been reported when Tovanor Breezhaler is administered with other medications used to treat COPD, such as rescue inhalers (e.g., salbutamol), methylxanthines (e.g., theophylline), and/or oral or inhaled steroids (e.g., prednisolone).
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
There are no data available on the use of this medication in pregnant women, and it is unknown whether the active ingredient of this medication passes into breast milk.
Driving and using machines
This medication is unlikely to affect your ability to drive or use machines.
Tovanor Breezhaler contains lactose
This medication contains lactose. If your doctor has told you that you have an intolerance to certain sugars, consult with them before taking this medication.
3. How to use Tovanor Breezhaler
Follow the instructions for administration of this medication exactly as indicated by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again.
How much Tovanor Breezhaler to use
The usual dose is to inhale the contents of one capsule each day.
You only need to inhale the medication once a day, as the effect of this medication lasts 24 hours.
Do not use more than the dose indicated by your doctor.
Elderly patients
If you are 75 years or older, you can use this medication at the same dose as other adults.
When to inhale Tovanor Breezhaler
Use this medication at the same time each day. This will help you remember to use it.
You can inhale this medication at any time before or after food or drink.
How to inhale Tovanor Breezhaler
- In this package, you will find an inhaler and capsules (in blisters) that contain the medication in powder form for inhalation. Use the capsules only with the inhaler provided in this package (Tovanor Breezhaler inhaler). The capsules should be kept in the blister until you need to use them.
- Do not press the capsule through the foil.
- When starting a new package, use the new Tovanor Breezhaler inhaler provided in the package.
- Discard the inhaler from each package once you have used all the capsules.
- Do not swallow the capsules.
- For more information on how to use the inhaler, please read the instructions at the end of this package leaflet.
If you use more Tovanor Breezhaler than you should
If you have inhaled too much of this medication or if someone uses your capsules accidentally, inform your doctor immediately or go to the nearest emergency center. Show the Tovanor Breezhaler package. Medical attention may be necessary.
If you forget to use Tovanor Breezhaler
If you forget to inhale a dose, inhale it as soon as possible. However, do not inhale two doses on the same day. Then, inhale the next dose at the usual time.
How long to continue treatment with Tovanor Breezhaler
- Continue using your treatment with this medication for as long as your doctor indicates.
- COPD is a long-term disease, and you should use this medication every day, not just when you have breathing problems or other symptoms of COPD.
Consult your doctor or pharmacist if you have any doubts about how long to continue treatment with this medication.
If you have any further questions on the use of this medication, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everybody gets them.
Some side effects can be serious but are rare
(may affect up to 1 in 100 patients)
- Irregular heartbeat
- High blood sugar levels (hyperglycemia: typical symptoms include excessive thirst or hunger and frequent urination)
- Skin rash, itching, hives, difficulty breathing or swallowing, dizziness (possible signs of an allergic reaction)
- Swelling, mainly of the tongue, lips, face, or throat (possible signs of angioedema)
If you experience any of these side effects, inform your doctor immediately.
Some side effects can be serious but the frequency is unknown
(cannot be estimated from the available data)
- Difficulty breathing with wheezing (high-pitched sounds when breathing) or coughing (signs of paradoxical bronchospasm)
Some side effects are common
(may affect up to 1 in 10 patients)
- Dry mouth
- Difficulty sleeping
- Runny or stuffy nose, sneezing, sore throat
- Diarrhea or abdominal pain
- Musculoskeletal pain
Some side effects are uncommon
(may affect up to 1 in 100 patients)
- Difficulty and pain when urinating
- Painful and frequent urination
- Palpitations
- Skin rash
- Numbness
- Coughing with sputum
- Tooth decay
- Pain or pressure in the cheeks and forehead
- Nosebleeds
- Pain in arms or legs
- Pain in the muscles, bones, or joints of the chest
- Stomach upset after meals
- Sore throat
- Fatigue
- Weakness
- Itching
- Voice changes (hoarseness)
- Nausea
- Vomiting
Some patients over 75 years of age experienced headache (frequently) and urinary tract infections (frequently).
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are side effects not listed in this package leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Tovanor Breezhaler
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date stated on the carton and blister after "EXP". The expiration date is the last day of the month indicated.
Do not store above 25°C.
Keep the capsules in the original blister to protect them from moisture. Remove them from the blister just before use.
The inhaler from each package should be discarded once you have used all the capsules.
Do not use this medication if you notice that the package is damaged or shows signs of deterioration.
Medications should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the package and any unused medication. This will help protect the environment.
6. Contents of the pack and additional information
Composition ofTovanor Breezhaler
- The active ingredient is glycopyrronium bromide. Each capsule contains 63 micrograms of glycopyrronium bromide (equivalent to 50 micrograms of glycopyrronium). The delivered dose (the dose delivered by the inhaler mouthpiece) is equivalent to 44 micrograms of glycopyrronium.
- The other ingredients of the powder for inhalation are lactose monohydrate and magnesium stearate.
Appearance of Tovanor Breezhaler and pack contents
The hard capsules of Tovanor Breezhaler 44 micrograms powder for inhalation are transparent and orange in color and contain a white powder. They have the product code «GPL50» printed in black above a black line and the company logo () printed in black below the same.
Each pack contains a device known as an inhaler, along with capsules in blisters. Each blister strip contains 6 or 10 hard capsules.
The following pack sizes are available:
Packs containing 6 x 1, 10 x 1, 12 x 1 or 30 x 1 hard capsules, along with an inhaler.
Multipack containing 90 (3 packs of 30 x 1) hard capsules and 3 inhalers.
Multipack containing 96 (4 packs of 24 x 1) hard capsules and 4 inhalers.
Multipack containing 150 (15 packs of 10 x 1) hard capsules and 15 inhalers.
Multipack containing 150 (25 packs of 6 x 1) hard capsules and 25 inhalers.
Not all pack sizes may be marketed.
Marketing authorisation holder
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublin 4
Ireland
Manufacturer
Novartis Farmacéutica SA
Gran Via de les Corts Catalanes, 764
08013 Barcelona
Spain
Novartis Pharma GmbH
Roonstraße 25
D-90429 Nuremberg
Germany
You can request more information about this medicinal product from the local representative of the marketing authorisation holder:
België/Belgique/Belgien Novartis Pharma N.V. Tel: +32 2 246 16 11 | Lietuva SIA Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
| Luxembourg/Luxemburg Novartis Pharma N.V. Tel: +32 2 246 16 11 |
Ceská republika Novartis s.r.o. Tel: +420 225 775 111 | Magyarország Novartis Hungária Kft. Tel.: +36 1 457 65 00 |
Danmark Novartis Healthcare A/S Tlf: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Deutschland Novartis Pharma GmbH Tel: +49 911 273 0 | Nederland Novartis Pharma B.V. Tel: +31 26 37 82 111 |
Eesti SIA Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Norge Novartis Norge AS Tlf: +47 23 05 20 00 |
Ελλάδα Novartis (Hellas) A.E.B.E. Τηλ: +30 210 28 11 712 INNOVIS PHARMA ΑΕΒΕ Τηλ: +30 210 66 64 805 | Österreich Novartis Pharma GmbH Tel: +43 1 86 6570 |
España Laboratorios Gebro Pharma, S.A. Tel: +34 93 205 86 86 | Polska Novartis Poland Sp. z o.o. Tel.: +48 22 375 4888 |
France Novartis Pharma S.A.S. Tél: +33 1 55 47 66 00 | Portugal Laboratório Medinfar - Produtos Farmacêuticos, S.A. Tel: +351 21 499 7400 |
Hrvatska Novartis Hrvatska d.o.o. Tel. +385 1 6274 220 | România Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Ireland Novartis Ireland Limited Tel: +353 1 260 12 55 | Slovenija Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Novartis Slovakia s.r.o. Tel: +421 2 5542 5439 |
Italia Alfasigma S.p.A. Tel: +39 06 91 39 4666 | Suomi/Finland Novartis Finland Oy Puh/Tel: +358 (0)10 6133 200 |
Κύπρος Novartis Pharma Services Inc. Τηλ: +357 22 690 690 | Sverige Novartis Sverige AB Tel: +46 8 732 32 00 |
Latvija SIA Novartis Baltics Tel: +371 67 887 070 | United Kingdom(Northern Ireland) Novartis Ireland LimitedTel: +44 1276 698370 |
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicinal product is available on the European Medicines Agency web site: http://www.ema.europa.eu
Instructions for use of the Tovanor Breezhaler inhaler
Read the Instructions for Usecompletely before using Tovanor Breezhaler | |||
|
|
|
|
Insert | Puncture and release | Inhale deeply | Check that the capsule is empty |
|
|
| |
|
|
|
|
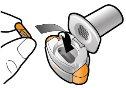
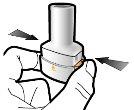
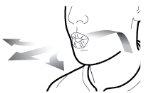
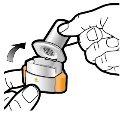
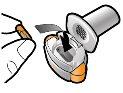
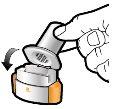
Step 1a: Remove the cap | Step 2a: Puncture the capsule once Hold the inhaler in a vertical position. Puncture the capsule by pressing both buttons firmly at the same time. | Step 3a: Exhale completely Do not blow into the inhaler. | Check that the capsule is empty Open the inhaler to check if there is any powder left in the capsule. |
| You should hear a sound when the capsule is punctured. Puncture the capsule only once. |
| If there is powder left in the capsule:
|
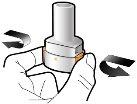
Step 1b: Open the inhaler |
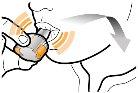
Step 2b: Release the buttons completely | Step 3b: Inhale the medicine deeply Hold the inhaler as shown in the figure. Put the mouthpiece in your mouth and close your lips firmly around it. Do not press the buttons. | Powder leftEmpty |
| Inspire quickly and as deeply as possible. You will hear a whirring sound during inhalation. You may notice the taste of the medicine when you inhale. |
| |
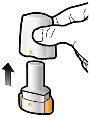
Step 1c: Remove the capsule Separate one of the blisters from the blister strip. Open the blister and remove a capsule. Do not press the capsule through the foil. Do not swallow the capsule. |
Step 3c: Hold your breath Hold your breath for 5 seconds. | Remove the empty capsule Discard the empty capsule in your household trash. Close the inhaler and put the cap back on. | |
Step 1d: Insert the capsule Never put the capsule directly into the mouthpiece. | Important information
| ||
Step 1e: Close the inhaler | |||
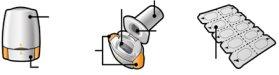
Your Tovanor Breezhaler pack contains:
| Frequently asked questions Why did the inhaler not make a sound when I inhaled? The capsule may be stuck in the compartment. If this happens, release the capsule carefully by tapping the base of the inhaler. Inhale the medicine again by repeating steps 3a to 3c. What should I do if there is powder left in the capsule? You have not received enough of your medicine. Close the inhaler and repeat steps 3a to 3c. I coughed after inhaling, is it important? It may happen. If the capsule is empty, it means you have received enough of your medicine. I notice small fragments of the capsule on my tongue, is it important? It may happen. It is not harmful. The likelihood of the capsules breaking into pieces increases if the capsule is punctured more than once. Cleaning the inhaler Wipe the mouthpiece from the inside and outside with a clean and dry cloth that does not leave lint to remove any powder residue. Keep the inhaler dry. Never wash your inhaler with water. | ||
Disposal of the inhaler after use Each inhaler should be discarded after all the capsules have been used. Ask your pharmacist how to dispose of medicines and inhalers that are no longer needed. |
- Country of registration
- Average pharmacy price47.61 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Tovanor Breezhaler 44 micrograms powder for inhalationDosage form: PULMONARY INHALATION, 50 microgramsActive substance: glycopyrronium bromideManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: PULMONARY INHALATION, 50 microgramsActive substance: glycopyrronium bromideManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: PULMONARY INHALATION, 0.0040 g/aerosolActive substance: ipratropium bromideManufacturer: Laboratorio Aldo Union S.L.Prescription required
Online doctors for Tovanor Breezhaler 44 micrograms powder for inhalation
Discuss questions about Tovanor Breezhaler 44 micrograms powder for inhalation, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions