
ENSPRYNG 120 mg READY-TO-USE INJECTION SOLUTION IN PRE-FILLED SYRINGE


How to use ENSPRYNG 120 mg READY-TO-USE INJECTION SOLUTION IN PRE-FILLED SYRINGE
Introduction
Package Leaflet: Information for the Patient
Enspryng 120 mg solution for injection in pre-filled syringe
satralizumab
This medicinal product is subject to additional monitoring, which will allow for the quick identification of new safety information. You can help by reporting any side effects you may get. The last section of the leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
In addition to this leaflet, your doctor will also give you a patient information card, which contains important safety information that you should know before and during treatment with Enspryng. Keep this information card with you at all times.
Contents of the pack
- What is Enspryng and what is it used for
- What you need to know before you use Enspryng
- How to use Enspryng
- Possible side effects
- Storage of Enspryng
- Contents of the pack and other information
Instructions for use
1. What is Enspryng and what is it used for
What is Enspryng
Enspryng contains the active substance satralizumab. It is a type of protein called a monoclonal antibody. Monoclonal antibodies are designed to recognize a specific substance in the body and bind to it.
What Enspryng is used for
Enspryng is a medicine used to treat neuromyelitis optica spectrum disorder (NMOSD) in adults and adolescents from 12 years of age.
What NMOSD is
NMOSD refers to a disease of the central nervous system that mainly affects the optic nerves and spinal cord. It is caused by the immune system (the body's defense) not working properly and attacking the body's nerves.
- Damage to the optic nerves causes inflammation, which in turn causes pain and vision loss.
- Damage to the spinal cord causes weakness or loss of mobility in the legs or arms, loss of sensation, and problems with bladder or bowel function.
In an NMOSD relapse, there is inflammation in the nervous system. This also happens when the disease recurs (relapses). The inflammation causes new symptoms or recurring previous symptoms.
How Enspryng works
Enspryng blocks the action of a protein called interleukin 6 (IL-6), which is involved in the process that leads to damage and inflammation in the nervous system. By blocking its effects, Enspryng reduces the risk of NMOSD relapse or flare.
2. What you need to know before you use Enspryng
Do not use Enspryng:
- if you are allergic to satralizumab or any of the other ingredients of this medicine (listed in section 6).
If you are in any of the above situations or are not sure, do not use Enspryng and consult your doctor, pharmacist, or nurse.
Warnings and precautions
If you experience any side effects, contact your doctor immediately (see section 4. Possible side effects).
Consult your doctor, pharmacist, or nurse before starting Enspryng if you are in any of the following situations (or if you are not sure).
Infections
You must not use Enspryng while you have an infection. Tell your doctor or nurse immediately if you think you have any signs of infectionbefore, during, or after treatment with Enspryng, such as:
- fever or chills
- persistent cough
- sore throat
- cold sores or genital ulcers (herpes simplex)
- shingles (herpes zoster)
- redness, swelling, sensitivity, or pain in the skin
- nausea or vomiting, diarrhea, or stomach pain.
You will also find this information on the patient information card that your doctor has given you. It is important that you have this information card with you at all times and show it to any doctor, nurse, or caregiver.
Your doctor will wait until the infection is under control before giving you Enspryng or allowing you to continue with Enspryng injections.
Vaccines
Tell your doctor if you have recently received any vaccineor may receive one in the near future.
- Your doctor will check if you need any vaccine before starting treatment with Enspryng.
- You must not receive live or attenuated live vaccines (e.g., BCG vaccine against tuberculosis or yellow fever vaccines) while being treated with Enspryng.
Liver enzymes
Enspryng may affect your liver and increase the amount of certain liver enzymes in your blood. Your doctor will perform blood tests before starting treatment with Enspryng and during treatment to check your liver function. Tell your doctor or nurse immediatelyif you have any of these signs of liver damage during or after treatment with Enspryng:
- yellowing of the skin and the white of the eyes (jaundice)
- dark-colored urine
- nausea and vomiting
- abdominal pain
White blood cell count
Your doctor will perform blood tests before starting treatment with Enspryng and during treatment to check your white blood cell count.
Children and adolescents
This medicine must not be given to children under 12 years of age, as it has not been studied in this age group yet.
Other medicines and Enspryng
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Tell your doctor or pharmacist if you are taking medicines such as warfarin, carbamazepine, phenytoin, and theophylline, as it may be necessary to adjust the dose.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Your doctor may recommend that you stop breastfeeding if you need to be treated with Enspryng. It is not known whether Enspryng passes into breast milk.
Driving and using machines
Enspryng is unlikely to affect your ability to drive, ride a bicycle, or use tools or machines.
3. How to use Enspryng
Follow exactly the instructions for administration of this medicine given by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again.
How much Enspryng to use
Each injection contains 120 mg of satralizumab. The first injection will be given under the supervision of your doctor or nurse.
- The first three injections are given once every 2 weeks. These are called the "loading doses".
- After that, the injection will be given every 4 weeks. This is called the "maintenance dose". You should continue with the injections every 4 weeks for as long as your doctor tells you.
How to use Enspryng
- Enspryng is given by injection under the skin (subcutaneously).
- For each injection, you must inject the entire contents of the syringe.
At first, your doctor or nurse may give you the Enspryng injection. However, your doctor may decide that you can give yourself the Enspryng injections or that an adult caregiver can give them to you.
- You or your caregiver will receive training to learn how to give the Enspryng injections.
- Talk to your doctor or nurse if you or your caregiver have any questions about giving the injections.
Read carefully and follow the "Instructions for use" at the end of this leaflet on how to inject Enspryng.
If you use more Enspryng than you should
Since Enspryng is in a pre-filled syringe, it is unlikely that you will receive too much. However, if you are concerned, consult your doctor, pharmacist, or nurse.
If you accidentally inject more doses than you should, contact your doctor. When you go to the doctor, always take the outer packaging with you.
If you forget to use Enspryng
It is very important to not miss any injections for the treatment to be fully effective.
If your doctor or nurse is giving you the injections and you miss an appointment, you must make another appointment as soon as possible.
If you are giving yourself the Enspryng injections and you miss one, give it as soon as possible. Do not wait until the next scheduled dose. After injecting the missed dose, you should give the next dose:
- if it is a loading dose: 2 weeks later
- if it is a maintenance dose: 4 weeks later
If you are unsure, consult your doctor, pharmacist, or nurse.
If you stop treatment with Enspryng
Do not stop treatment with Enspryng suddenly without consulting your doctor first. If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Allergic reaction
Tell your doctor immediately or go to the emergency department of your nearest hospital if you have any signs of an allergic reaction during or after the injection. For example:
- chest tightness or wheezing
- feeling of difficulty breathing
- fever or chills
- severe dizziness or feeling of fainting
- swelling of the lips, tongue, or face
- itching of the skin, hives, or rash.
Do not give yourself the next dose until you have spoken to your doctor and they tell you that you can.
Injection-related reactions(very common: may affect more than 1 in 10 people)
In most cases, these are mild reactions, although some can be serious.
Tell your doctor or nurse immediately if you have any of these signs during or after the injection, especially in the first 24 hours after the injection:
- redness, itching, pain, or swelling at the injection site
- rash, redness, or itching of the skin or hives
- flushing
- headache
- irritation, swelling, or pain in the throat
- feeling of difficulty breathing
- low blood pressure (dizziness and feeling of fainting)
- fever or chills
- feeling of tiredness
- nausea, vomiting, or diarrhea
- fast heart rate, rapid heartbeat, or increased pulse (palpitations).
Tell your doctor or nurse immediately if you have any of the above symptoms.
Other side effects:
Very common(may affect more than 1 in 10 people):
- headache
- joint pain
- high levels of lipids in the blood (fats)
- low white blood cell count
Common(may affect up to 1 in 10 people):
- feeling of stiffness
- migraine
- slow heart rate (bradycardia)
- high blood pressure
- difficulty sleeping
- swelling in the lower legs, feet, or hands
- rash or itching
- allergies or allergic rhinitis
- stomach inflammation (gastritis), including stomach pain and nausea
- weight gain
- blood tests showing:
- low levels of fibrinogen (a protein involved in blood clotting)
- high levels of liver enzymes (transaminases, possible sign of liver problems)
- high levels of bilirubin (possible sign of liver problems)
- low platelet count (which can cause easy bleeding and bruising)
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Enspryng
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the label of the pre-filled syringe and on the carton after "EXP". The expiry date refers to the last day of the month shown.
- Store in a refrigerator (between 2°C and 8°C). Do not freeze. Do not use the syringe if it has been frozen. Always keep the syringe dry.
- Store the pre-filled syringes in the outer carton to protect them from light and moisture.
- If stored in the outer carton and unopened, Enspryng can be kept out of the refrigerator for a single period of up to 8 days at temperatures below 30°C. Do not put Enspryng back in the refrigerator.
- You must not use and must discard the pre-filled syringe if it has been out of the refrigerator for more than 8 days.
Do not use this medicine if you notice that it is cloudy, has changed color, or contains particles. Enspryng is a colorless to slightly yellowish liquid.
The medicine must be injected immediately after removing the needle cap and before 5 minutes to avoid the medicine drying out and blocking the needle. If the pre-filled syringe is not used within 5 minutes after removing the needle cap, it must be discarded in a puncture-resistant container and a new one used.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Enspryng Composition
- The active ingredient is satralizumab. Each pre-filled syringe contains 120 mg of satralizumab in 1 ml.
- The other components are histidine, aspartic acid, arginine, poloxamer 188, and water for injectable preparations.
Product Appearance and Container Contents
- It is a colorless to slightly yellowish liquid.
- Enspryng is an injectable solution.
- Each Enspryng container contains 1 pre-filled syringe. Each Enspryng multiple container contains 3 pre-filled syringes (3 containers of 1). Only certain container sizes may be marketed.
Marketing Authorization Holder
Roche Registration GmbH
Emil-Barell-Strasse 1
79639 Grenzach-Wyhlen
Germany
Manufacturer
Roche Pharma AG
Emil-Barell-Strasse 1
79639 Grenzach-Wyhlen
Germany
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien N.V. Roche S.A. Tél/Tel: +32 (0) 2 525 82 11 | Lietuva UAB “Roche Lietuva” Tel.: +370 5 2546799 |
| Luxembourg/Luxemburg (See Belgique/Belgien) |
Ceská republika Roche s. r. o. Tel.: +420 - 2 20382111 | Magyarország Roche (Magyarország) Kft. Tel.: +36 - 1 279 4500 |
Danmark Roche Pharmaceuticals A/S Tel.: +45 - 36 39 99 99 | Malta (See Ireland) |
Deutschland Roche Pharma AG Tel.: +49 (0) 7624 140 | Nederland Roche Nederland B.V. Tel.: +31 (0) 348 438050 |
Eesti Roche Eesti OÜ Tel.: + 372 - 6 177 380 | Norge Roche Norge AS Tel.: +47 - 22 78 90 00 |
Ελλάδα Roche (Hellas) A.E. Tel.: +30 210 61 66 100 | Österreich Roche Austria GmbH Tel.: +43 (0) 1 27739 |
España Roche Farma S.A. Tel: +34 - 91 324 81 00 | Polska Roche Polska Sp.z o.o. Tel.: +48 - 22 345 18 88 |
France Roche Tel: +33 (0) 1 47 61 40 00 | Portugal Roche Farmacêutica Química, Lda Tel.: +351 - 21 425 70 00 |
Hrvatska Roche d.o.o. Tel.: +385 1 4722 333 | România Roche România S.R.L. Tel.: +40 21 206 47 01 |
Ireland Roche Products (Ireland) Ltd. Tel.: +353 (0) 1 469 0700 | Slovenija Roche farmacevtska družba d.o.o. Tel.: +386 - 1 360 26 00 |
Ísland Roche Pharmaceuticals A/S c/o Icepharma hf Sími: +354 540 8000 | Slovenská republika Roche Slovensko, s.r.o. Tel.: +421 - 2 52638201 |
Italia Roche S.p.A. Tel.: +39 - 039 2471 | Suomi/Finland Roche Oy Puh/Tel: +358 (0) 10 554 500 |
Kύπρος Γ.Α.Σταμάτης & Σια Λτδ. Tel.: +357 - 22 76 62 76 | Sverige Roche AB Tel.: +46 (0) 8 726 1200 |
Latvija Roche Latvija SIA Tel.: +371 - 6 7039831 | United Kingdom (Northern Ireland) Roche Products (Ireland) Ltd. Tel: +44 (0) 1707 366000 |
Date of Last Revision of this Leaflet:
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
There are also links to other websites about rare diseases and orphan medicines.
Instructions for Use
Read these instructions for use:
- Before starting to use the pre-filled syringe
- Each time you are dispensed the prescribed medication, in case it contains new information.
- This information does not replace consultation with your doctor or nurse about your disease or treatment.
- Your doctor or nurse will decide if you can administer Enspryng injections yourself at home or if a caregiver can do it. They will also show you or your caregiver the correct and safe way to use the syringe before using it for the first time.
- In case of doubt, consult your doctor or nurse.
Important Information
- Each syringe is pre-filled with a medication called Enspryng.
- Each Enspryng container contains only 1 pre-filled syringe.
- Each pre-filled syringe can only be used once.
- Do not share your syringes with other people.
- Do not remove the needle cap until you are ready to administer the Enspryng injection.
- Do not use the syringe if it has been dropped or is damaged.
- Do not attempt to disassemble the syringe at any time.
- Do not leave the syringe unattended.
- Do not reuse the same syringe.
Materials Needed for Injection
Each Enspryng container contains:
- 1 single-use pre-filled syringe.
You will also need the following, which are not included in the container:

- 1 alcohol swab
- 1 cotton ball or sterile gauze
- 1 plaster
- 1 puncture-resistant container for needles to safely dispose of the used needle cap and syringe. See step 21 "How to Dispose of Enspryng" at the end of these instructions for use.
Enspryng Pre-filled Syringe
(see Figures A and B)
Before Use:
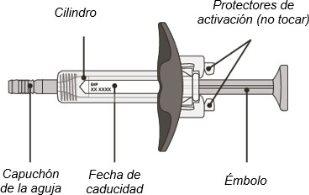
Figure A
After Use:
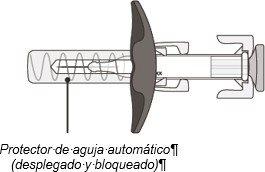
Figure B
The syringe has an automatic needle protector that covers the needle when the injection is complete.
Prepare for Enspryng Administration
- Remove the container containing the syringe from the refrigerator and place it on a clean and flat work surface (such as a table).
- Check the expiration date on the back of the container (see Figure C). Do notuse the syringe if the container has expired.
- Check that the front of the container is properly sealed (see Figure C). Do notuse the syringe if the container's security seal is broken.
If the expiration date has passed or the seal is broken, go to step 21 "How to Dispose of Enspryng" and contact your doctor or nurse.
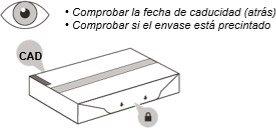
Figure C
- Open the sealed container (see Figure D).
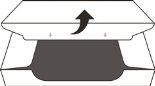
Figure D
- Carefully remove the syringe from the container by holding the cylinder (see Figure E).
- Do not turn the container upside down to remove the syringe.
- Do not touch the activation protectors, as this may damage the syringe.
- Do not hold the syringe by the plunger or the needle cap.
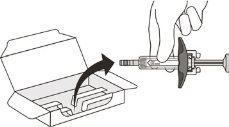
Figure E
Examine the Syringe
(see Figure F)
- Check the expiration date of the syringe. Do notuse the syringe if it has expired.
- Inspect the syringe for damage. Do notuse it if it is cracked or broken.
- Through the viewing window, check that the liquid is clear and colorless to slightly yellowish.
Do notinject the medication if the liquid is cloudy, has changed color, or contains particles.
- There may be small air bubbles in the syringe. This is normal and should not be attempted to be removed.
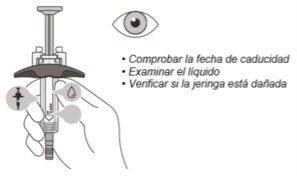
Figure F
If the expiration date has passed, the syringe is damaged, or the liquid is cloudy, has changed color, or contains particles, do not use the medication. Then, go to step 21 "How to Dispose of Enspryng" and contact your doctor or nurse.
Allow the Syringe to Reach Room Temperature
- Once you have examined the syringe, place it on a clean and flat work surface (such as a table) for 30 minutesto allow it to reach room temperature (see Figure G).
It is important to allow the syringe to gently reach room temperature, as cold medication can be uncomfortable and it is more difficult to push the plunger.
- Do not accelerate the warming process by heating the syringe in any way.
- Do not remove the needle cap while waiting for the syringe to reach room temperature.

Figure G
Wash Your Hands
- Wash your hands with water and soap (see Figure H).
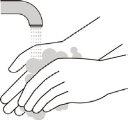
Figure H
Choose the Injection Site
- Choose an injection site from the following:
- the lower abdomen or
- the front and middle of the thighs (see Figure I).
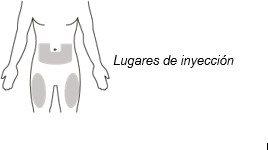
Figure I
- Do not inject into the 5 cm around the navel.
- Do not inject into moles, scars, bruises, or areas where the skin is sensitive, red, hard, or damaged.
Choose a different injection site for each new injection. Choose a site that is at least 2.5 cm away from the site used last time.
Clean the Injection Site
- Clean the injection site with an alcohol swab and let it air dry.
- Do not fan or blow on the cleaned area.
- Do not touch the injection site again before administering the injection.
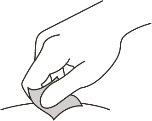
Figure J
How to Inject Enspryng
- Hold the syringe cylinder with your thumb and index finger. With your other hand, pull the needle cap straight off. You may see a drop of liquid at the end of the needle; this is normal and will not affect the dose (see Figure K).
- Use the syringe within 5 minutes of removing the needle cap; otherwise, the needle may become clogged.
- Do not remove the needle cap until you are ready to administer the Enspryng injection.
- Do not replace the needle cap once it has been removed, as this may damage the needle.
- Do not touch the needle or let it touch any surface after the cap has been removed.
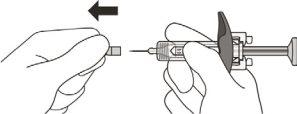
Figure K
- Immediately discard the needle cap in a puncture-resistant container for needle disposal. See step 21 "How to Dispose of Enspryng".
- Hold the syringe cylinder with your thumb and index finger. With your other hand, pinch the cleaned skin (see Figure L).
- With a quick motion, like throwing a dart, insert the needle into the skin at an angle of 45° to 90° (see Figure L).
- Do not change the injection angle while administering the injection.
- Do not reinsert the needle.
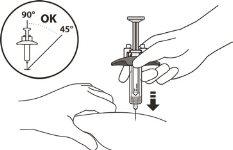
Figure L
- Once the needle is inserted, gently release the pinched skin.
- Slowly inject all of the medication by gently pushing the plunger down until it reaches the activation protectors (see Figure M).
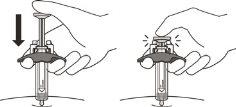
Figure M
- Carefully release the plunger and let the needle come out of the skin, keeping the same angle as when it was inserted (see Figure N).
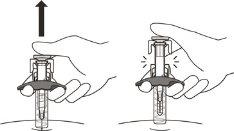
Figure N
- Then, the automatic needle protectorwill cover the needle. If the needle is not covered, carefully place the syringe in a puncture-resistant container for needle disposal to avoid injury. See step 21 "How to Dispose of Enspryng".
Care of the Injection Site
- There may be a little bleeding at the injection site. You can press the injection site with a cotton ball or sterile gauze until the bleeding stops, but do not rub. If necessary, you can also cover the injection site with a plaster. If the medication comes into contact with the skin, wash the area with water.
How to Dispose of Enspryng
- Do not attempt to replace the needle cap on the syringe. Place the used syringe in a puncture-resistant container for needle disposal immediately after use (see Figure O). Do not throw the syringe in your household trash or recycle it.
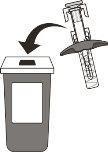
Figure O
- Ask your doctor, nurse, or pharmacist where you can get a "sharps" container or what other type of puncture-resistant container you can use to safely dispose of used syringes and needle caps.
- Dispose of the entire puncture-resistant container for needle disposal as instructed by your doctor or pharmacist.
- Do not throw the puncture-resistant container for needle disposal in your household trash.
- Do not recycle the used puncture-resistant container for needle disposal.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ENSPRYNG 120 mg READY-TO-USE INJECTION SOLUTION IN PRE-FILLED SYRINGEDosage form: INJECTABLE PERFUSION, 130 mgActive substance: ustekinumabManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 45 mgActive substance: ustekinumabManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 90 mgActive substance: ustekinumabManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for ENSPRYNG 120 mg READY-TO-USE INJECTION SOLUTION IN PRE-FILLED SYRINGE
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for ENSPRYNG 120 mg READY-TO-USE INJECTION SOLUTION IN PRE-FILLED SYRINGE – subject to medical assessment and local rules.






