
ENEMA CASEN 139 mg/ml + 32 mg/ml RECTAL SOLUTION

How to use ENEMA CASEN 139 mg/ml + 32 mg/ml RECTAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET: INFORMATION FOR THE USER
Enema Casen 139 mg/mL and 32 mg/mL rectal solution
sodium dihydrogen phosphate anhydrous/disodium hydrogen phosphate anhydrous
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What is Enema Casen and what is it used for
- Before you use Enema Casen
- How to use Enema Casen
- Possible side effects
- Storing Enema Casen
- Contents of the pack and further information
1. What is Enema Casen and what is it used for
Enema Casen belongs to a group of medicines called rectal laxatives.
Enema Casen is indicated in cases where intestinal evacuation is necessary, such as:
- Pre and post-surgery.
- Childbirth and post-childbirth.
- Before rectoscopy and sigmoidoscopy (examination of the intestine by endoscopic techniques using a probe).
- Before radiological examinations
- Fecal impaction.
You should consult a doctor if it worsens or does not improve after 6 days
2. What you need to know before you start using Enema Casen
Do not use Enema Casen
- If you are allergic to sodium dihydrogen phosphate anhydrous, disodium hydrogen phosphate anhydrous, or any of the other components of this medicine (listed in section 6).
- In children under 2 years of age.
- If there is suspicion of intestinal obstruction.
- If you have megacolon (colon dilation), ileostomy (artificial anus), anorectal stenosis (narrowing of the rectum), inability to produce normal bowel movements (paralytic ileus), or imperforate anus.
- Congenital obstruction of the colon (Hirschsprung's disease).
- If you have severe or moderate kidney failure.
- If you have symptomatic heart failure.
- If you have symptoms of appendicitis, intestinal perforation, or active inflammatory bowel disease (such as Crohn's disease or ulcerative colitis).
- If you have undiagnosed rectal bleeding.
- If you have uncontrolled high blood pressure.
- If you are dehydrated.
In case of doubt about any of these situations or your particular situation, consult your doctor.
Warnings and precautions
Consult your doctor or pharmacist before using Enema Casen:
- If after 10 minutes of administration of the enema, you do not manage to evacuate (evacuation occurs approximately 5 minutes after administration), it is recommended to consult your doctor so that they can perform the necessary tests to minimize the risk of serious hyperphosphatemia.
- If you are an elderly patient, weak, suffering from ascites (fluid accumulation in the abdomen), anal ulcers or fissures, or suffering from kidney or heart disease.
- If you have had a colostomy (major colon surgery), you should consult your doctor.
- If you have pre-existing electrolyte imbalances (alteration of mineral salt levels in the body) as hypocalcemia (decreased calcium levels in the blood), hypokalemia (decreased potassium levels in the blood), hyperphosphatemia (increased phosphorus levels in the blood), hypernatremia (increased sodium levels in the blood), dehydration, and acidosis may occur.
- In case of suspected electrolyte disorders, your doctor will perform a blood test to determine the levels of these substances before administering Enema Casen.
- If you have nausea, vomiting, or abdominal pain, you should follow your doctor's instructions.
- Repeated and prolonged use of Enema Casen is not recommended as it may cause habituation. Do not use if symptoms worsen or persist without consulting a doctor.
- Enema Casen should not be used for more than 6 consecutive days, unless indicated by a doctor.
- Enema Casen should be administered following the instructions for use and handling. You should interrupt administration if you encounter resistance, as forcing administration can cause injuries.
- If you bleed from the rectum after administration of the enema, do not administer more enemas and consult your doctor immediately.
- Consult your doctor if signs of irritation occur.
This medicine should not be used as a habitual treatment for constipation.
Using Enema Casen with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
It is especially important that your doctor knows if you are taking:
- medicines for high blood pressure (hypertension), such as angiotensin-converting enzyme inhibitors (e.g., enalapril, ramipril, lisinopril) and angiotensin receptor antagonists (e.g., losartan, candesartan, eprosartan, irbesartan, olmesartan, telmisartan, valsartan),
- medicines for angina pectoris (calcium channel blockers),
- medicines to empty the bladder (diuretics),
- medicines for behavioral disorders (lithium),
- medicines that may alter water or electrolyte balance (potassium, sodium, phosphate, calcium) in the blood,
- medicines for intestinal cleansing (sodium phosphate),
- medicines that alter heart rhythm (prolong QT interval): such as amiodarone, arsenic trioxide, astemizole, azithromycin, erythromycin, clarithromycin, chlorpromazine, cisapride, citalopram, domperidone, terfenadine, procainamide.
- non-steroidal anti-inflammatory medicines (NSAIDs) such as aspirin or ibuprofen.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
If you are breastfeeding, you should express and discard the milk produced during at least the 24 hours following administration of Enema Casen.
Driving and using machines
Enema Casen will not affect your ability to drive or use machines.
Enema Casen contains benzalkonium chloride
This medicine contains 0.6 mg of benzalkonium chloride per milliliter of rectal solution.
Benzalkonium chloride may cause local irritation.
3. How to use Enema Casen
Follow the instructions for administration of Enema Casen as indicated by your doctor. Consult your doctor or pharmacist if you have doubts.
Enema Casen is a rectal medicine. It should be administered at room temperature. For self-administration of Enema Casen, it is recommended that the patient lie on their left side and with both legs bent over the chest (Figure 1) or lie with the left leg extended and the right leg bent over the chest (Figure 2).
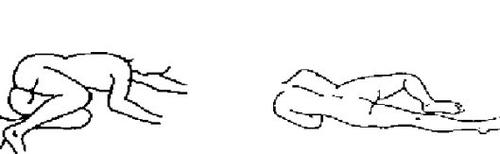 Figure 1 Figure 2
Figure 1 Figure 2
When Enema Casen is to be administered to the patient by another person, the recommended positions may be either those described for self-administration or the one shown in Figure 3.
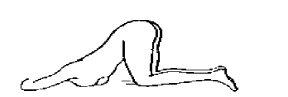
Figure 3
Remove the blue protective sleeve from the pre-lubricated cannula.
In the indicated positions, carefully insert the cannula into the rectum to avoid damaging the wall and press the container in a smooth and continuous manner until the required amount of liquid is introduced. It is advisable for the patient to maintain this position until they feel strong urges to defecate.
Generally, 2 to 5 minutes are sufficient to obtain the desired effect. If the product is not expelled after this time, see section 4: "Possible side effects".
In general, the following dose is recommended:
Children
- Infants and children under 2 years: Do not administer Enema Casen in children under two years, its use is contraindicated.
- Children from 2 to 15 years: The recommended dose is a single dose of 5 mL/kg, or up to a maximum of 140 mL. The maximum duration of treatment will be one enema per day, for no more than 6 consecutive days.
Adults
The recommended dose is a single enema of 140 mL or 250 mL. It can be administered once a day, for a maximum of 6 consecutive days.
Elderly
The recommended dosage is the same as for adults.
Patients with altered liver function
In this case, no dose adjustment is necessary.
Patients with altered kidney function
This medicine should not be administered to patients with severe or moderate kidney failure.
It will be administered with caution to patients with mild kidney failure and only under medical prescription.
If you use more Enema Casen than you should
Consult your doctor or pharmacist immediately or the toxicology information service. Phone: 91 562 04 20.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects are described for Enema Casen according to their frequency:
Very rare side effects (may affect up to 1 in 10,000 people):
- allergic reaction,
-tetany (painful muscle spasms of the limbs), hyperphosphatemia (increased phosphorus in the blood), dehydration,
-hypocalcemia (decreased calcium in the blood), hypokalemia (decreased potassium in the blood), hypernatremia (increased sodium in the blood), metabolic acidosis (accumulation of acids in body fluids), nausea, vomiting, abdominal pain, abdominal distension (swollen abdomen), diarrhea, gastrointestinal pain, anal pain and discomfort, itching, blisters, itching, rectal irritation, and chills.
Very common side effects (may affect more than 1 in 10 people):
- Transient elevation of phosphorus in the blood.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Enema Casen
It does not require special storage conditions, however, in rare cases, some inorganic flocculations may appear in the solution that do not affect the integrity of the preparation.
Keep out of the reach and sight of children.
Do not use Enema Casen after the expiration date stated on the packaging. The expiration date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medicines in the pharmacy's SIGRE collection point. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Contents of the pack and further information
Composition of Enema Casen
- The active ingredients are sodium dihydrogen phosphate anhydrous and disodium hydrogen phosphate anhydrous. Each milliliter of rectal solution contains 139 mg of sodium dihydrogen phosphate anhydrous and 32 mg of disodium hydrogen phosphate anhydrous.
- The other ingredients are benzalkonium chloride, disodium edetate, and purified water.
Appearance of the product and pack contents
Enema Casen is presented as a colorless and odorless rectal solution, in flexible plastic containers of 250, 140, and 80 mL, hermetically sealed, with an anti-return valve and a pre-lubricated rectal cannula free of latex.
Marketing authorization holder and manufacturer
Casen Recordati, S.L.
Autovía de Logroño, km. 13,300
50180 Utebo (Zaragoza).
Date of last revision of this leaflet: October 2017
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ENEMA CASEN 139 mg/ml + 32 mg/ml RECTAL SOLUTIONDosage form: RECTAL LIQUID, PER 100 ml: 14/3 gActive substance: sodium phosphateManufacturer: Lainco S.A.Prescription requiredDosage form: RECTAL LIQUID, 6.14 ml glycerolActive substance: glycerolManufacturer: Casen Recordati S.L.Prescription not requiredDosage form: RECTAL LIQUID, 450 mg / 45 mgActive substance: sodium lauryl sulfoacetate, incl. combinationsManufacturer: Lainco S.A.Prescription not required
Online doctors for ENEMA CASEN 139 mg/ml + 32 mg/ml RECTAL SOLUTION
Discuss questions about ENEMA CASEN 139 mg/ml + 32 mg/ml RECTAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















