
DYMISTA 137 micrograms/50 micrograms/application NASAL SPRAY SUSPENSION


How to use DYMISTA 137 micrograms/50 micrograms/application NASAL SPRAY SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Dymista
137 micrograms/50 micrograms/dose,
nasal spray suspension
Azelastine hydrochloride / Fluticasone propionate
Read this package leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
- What Dymista is and what it is used for.
- What you need to know before you use Dymista.
- How to use Dymista.
- Possible side effects.
- How to store Dymista.
- Contents of the pack and other information.
1. What Dymista is and what it is used for
Dymista contains two active substances: azelastine hydrochloride and fluticasone propionate.
- Azelastine hydrochloride belongs to a group of medicines called antihistamines. Antihistamines work by preventing the effects of substances such as histamine, which is produced by the body as part of an allergic reaction; this reduces the symptoms of allergic rhinitis.
- Fluticasone propionate belongs to a group of medicines called corticosteroids, which reduce inflammation.
Dymista is used to relieve the symptoms of moderate to severe seasonal allergic rhinitis and perennial allergic rhinitis, if treatment with an antihistamine or intranasal corticosteroid alone is considered insufficient.
Seasonal or perennial allergic rhinitis are allergic reactions to substances such as pollen (hay fever), house dust mites, molds, dust, or domestic animals.
Dymista relieves the symptoms of allergies, such as runny nose, postnasal drip, sneezing, itching, or nasal congestion.
2. What you need to know before you use Dymista
Do not use Dymista:
If you are allergic to azelastine hydrochloride or fluticasone propionate, or to any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor or pharmacist before using Dymista if:
You have recently had nasal surgery.
You have a nasal infection. Nasal infections should be treated with antibiotics or antifungals. If you have been given medication for a nasal infection, you can continue using Dymista to treat your allergy.
You have tuberculosis or an untreated infection.
You have changes in vision or a history of increased intraocular pressure, glaucoma, and/or cataracts. If you have any of these conditions, you will be closely monitored during the use of Dymista.
You have impaired adrenal function. Caution should be exercised when switching from systemic steroid treatment to Dymista.
You have severe liver disease. The risk of systemic adverse effects will increase.
In these cases, your doctor will decide if you can use Dymista.
It is essential that you take the dose indicated in section 3 or as indicated by your doctor. Treatment with higher doses of nasal corticosteroids than recommended may lead to adrenal suppression, which can cause weight loss, fatigue, muscle weakness, low blood sugar levels, salt cravings, joint pain, depression, and skin darkening. If you experience any of these side effects, your doctor may recommend another medication during periods of stress or elective surgery.
To avoid adrenal suppression, your doctor will advise you to take the lowest dose that maintains effective control of your rhinitis symptoms.
The use of nasal corticosteroids (such as Dymista) may cause slower growth in children and adolescents when used for a long period. The doctor will regularly monitor the growth of children and ensure they take the lowest effective dose possible.
Contact your doctor if you experience blurred vision or other visual disturbances.
If you are not sure if you are in any of the above situations, consult your doctor or pharmacist before using Dymista.
Children
This medicine is not recommended for use in children under 12 years of age.
Using Dymista with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines, including those obtained without a prescription.
Some medicines may increase the effects of Dymista, so your doctor will closely monitor you if you are taking these medicines (including some for HIV: ritonavir, cobicistat, and medicines for the treatment of fungal infections: ketoconazole).
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Driving and using machines
Dymista has a minimal influence on the ability to drive and use machines.
Very rarely, you may experience fatigue or dizziness due to the disease itself or during the use of Dymista. In these cases, do not drive or operate machinery. Note that alcohol consumption may enhance these effects.
Dymista contains benzalkonium chloride.
This medicine contains 14 micrograms of benzalkonium chloride per spray.
This medicine may cause irritation or inflammation of the nasal mucosa, especially with long-term treatments, because it contains benzalkonium chloride. If such a reaction is suspected (persistent nasal congestion), a nasal medicine that does not contain this excipient should be used whenever possible.
3. How to use Dymista
Follow the instructions for administration of this medicine exactly as indicated by your doctor. If you are unsure, consult your doctor or pharmacist again.
It is essential that the use of Dymista is regular for the therapeutic benefit to be complete.
Avoid contact with the eyes.
Adults and adolescents (over 12 years)
The recommended dose is one spray in each nostril, in the morning and in the evening.
Use in children under 12 years
This medicine is not recommended for use in children under 12 years of age.
Use in case of renal and hepatic insufficiency
No data are available in patients with renal and hepatic insufficiency.
Method of administration
Nasal route.
Read the following instructions carefully and use the product only as indicated.
INSTRUCTIONS FOR USE
Preparing the spray
- Gently shake the bottle for 5 seconds, tilting it from top to bottom, and then remove the protective cap (see figure 1).
Figure 1

- The first time you use the nasal spray, you need to prime the pump by spraying into the air.
- Prime the pump by placing two fingers on either side of the spray pump and your thumb on the bottom of the bottle.
- Pump and release the pump 6 times, until a fine spray appears (see figure 2).
- The pump is now primed and ready for use.
Figure 2
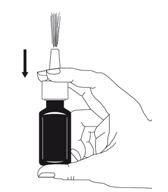
- If you have not used the nasal spray for more than 7 days, you will need to re-prime the pump by pressing and releasing it.
Spraying method
- Gently shake the bottle for 5 seconds, tilting it from top to bottom, and then remove the protective cap (see figure 1).
- Blow your nose to clear your nostrils.
- Tilt your head forward. Do not tilt your head back.
- Hold the bottle upright and carefully insert the tip of the spray pump into one nostril. 5. Close the other nostril with your finger, pump the spray quickly once, and at the same time, breathe in gently (see figure 3).
- Breathe through your mouth.
Figure 3

- Repeat the operation in the other nostril.
- Breathe gently and do not tilt your head back after application. This will prevent the medicine from reaching your throat, causing an unpleasant taste (see figure 4).
Figure 4

- After each use, clean the tip of the spray pump with a tissue or a clean cloth, and replace the protective cap.
- Do not poke the nozzle in case the spray is not obtained. Clean the valve with water.
It is essential that you use the prescribed dose. Use only the amount recommended by your doctor.
Duration of treatment
Dymista is suitable for prolonged use. The duration of treatment corresponds to the period during which you experience allergic symptoms.
If you use more Dymista than you should
If you accidentally spray more of this medicine into your nose, it is unlikely to cause any problems. If you are unsure or have used a higher dose than recommended for a long time, consult your doctor. If a person, especially a child, accidentally swallows Dymista, consult your doctor immediately or go to the nearest medical center, or call the Toxicology Information Service. Phone (91) 562 04 20, indicating the medicine and the amount ingested.
If you forget to use Dymista
Use the nasal spray as soon as you remember and then take your next dose at the usual time. Do not take a double dose to make up for forgotten doses.
If you stop using Dymista
Do not stop treatment without consulting your doctor, as this may put your treatment at risk.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Dymista can cause side effects, although not everybody gets them.
Very common side effects (may affect more than 1 in 10 people):
- Nasal bleeding.
Common side effects (may affect up to 1 in 10 people):
- Headache.
- Bitter taste in the mouth, especially if you tilt your head back during the use of the nasal spray. This taste should disappear if you drink a soft drink a few minutes after using this medicine.
- Unpleasant smell.
Uncommon side effects (may affect up to 1 in 100 people):
- Mild irritation of the inside of the nose, which can cause mild itching, tingling, or sneezing.
- Nasal dryness, cough, dry throat, or throat irritation.
Rare side effects (may affect up to 1 in 1,000 people):
- Dry mouth.
Very rare side effects (may affect up to 1 in 10,000 people):
- Dizziness or drowsiness.
- Cataracts, glaucoma, or increased pressure in the eye, with possible loss of vision and/or redness and eye pain. These side effects have been observed with prolonged treatment with nasal sprays of fluticasone propionate.
- Skin and mucous membrane lesions of the nose.
- Feeling unwell, tiredness, exhaustion, or weakness.
- Rash, redness, or itching of the skin, hives.
- Bronchospasm (narrowing of the airways in the lungs).
Seek immediate medical attention if you develop any of the following symptoms:
- Swelling of the face, lips, tongue, or throat, which may cause difficulty swallowing/breathing and sudden appearance of a rash on the skin.These could be signs of a severe allergic reaction. Note that this is very rare.
Side effects with unknown frequency (frequency cannot be estimated from the available data)
- Blurred vision
- Nasal ulcers
When this medicine is administered at high doses for a prolonged period, systemic side effects (side effects that affect the whole body) may occur. The likelihood of these effects is much lower if a nasal corticosteroid is used instead of oral corticosteroids. These effects may vary between individual patients and between different corticosteroid preparations (see section 2).
Nasal corticosteroids may affect the normal production of hormones in your body, especially if they are used at high doses for a long time. In children and adolescents, this side effect may cause slower growth.
In rare cases, a reduction in bone density (osteoporosis) may occur when corticosteroids are administered nasally for a long period.
Reporting of side effects:
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Dymista
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the bottle and carton after “EXP”. The expiry date is the last day of the month stated.
Do not refrigerate or freeze.
Shelf life after opening: Discard any unused medicine 6 months after opening the nasal spray for the first time.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Package Contents and Additional Information.
Composition of Dymista
The active ingredients are: azelastine hydrochloride and fluticasone propionate.Each gram of suspension contains 1,000 micrograms of azelastine hydrochloride and 365 micrograms of fluticasone propionate.
Each application (0.14 g) releases 137 micrograms of azelastine hydrochloride (= 125 micrograms of azelastine) and 50 micrograms of fluticasone propionate.
The other components are: disodium edetate, glycerol, microcrystalline cellulose, sodium carmellose, polysorbate 80, benzalkonium chloride, phenylethyl alcohol, and purified water.
Appearance of the Product and Package Contents
Dymista is a homogeneous white suspension.
Dymista is presented in an amber glass bottle, equipped with a spray pump, an applicator, and a protective cap.
The 10 ml bottle contains 6.4 g of nasal spray suspension (at least 28 applications). The 25 ml bottle contains 23 g of nasal spray suspension (at least 120 applications).
Dymista has the following presentations:
Package with a bottle containing 6.4 g of nasal spray suspension.
Package with a bottle containing 23 g of nasal spray suspension.
Multiple package containing 10 bottles with 6.4 g each of nasal spray suspension.
Multiple package containing 3 bottles with 23 g each of nasal spray suspension.
Not all package sizes may be marketed.
Marketing Authorization Holder
Viatris Healthcare Limited
Damastown Industrial Park
Mulhuddart, Dublin 15
Dublin
Ireland
Manufacturer
Mylan Hungary Kft,
H-2900 Komárom,
Mylan utca 1,
Hungary
or
MEDA Pharma GmbH & Co. KG
Benzstrasse 1
61352 Bad Homburg
(Germany)
or
Haupt Pharma Amareg GmbH
Donaustaufer Str. 378
93055 Regensburg
(Germany)
or
Madaus GmbH
Lütticher Straße 5
53842 Troisdorf
Germany
For further information about this medicinal product, please contact the local representative of the marketing authorization holder:
Viatris Pharmaceuticals, S.L.
C/ General Aranaz, 86
28027 - Madrid
Spain
This medicinal product is authorized in the Member States of the European Economic Areaand in the United Kingdom (Northern Ireland)under the following names:
Austria Dymista Nasenspray
Bulgaria Dymista
Cyprus Dymista Ρινικ? εκν?φωμα
Czech Republic Dymistin 137 mikrogramu / 50 mikrogramu, nosní sprej, suspenze
Denmark Dymista
Estonia Dymista
Finland Dymista nenäsumute
France Dymistalin Suspension pour pulvérisation nasale
Germany Dymista Nasenspray 137 Mikrogramm/50 Mikrogramm pro SprühstoßNasenspray, Suspension
Greece Dymista Ρινικ? εκν?φωμα
Hungary Dymista Szuszpenziós orrspray
Iceland Dymista Nefúði
Ireland Dymista Nasal Spray
Italy Dymista
Latvia Dymista 137 mikrogrami/50 mikrogrami deva deguna aerosols, suspensija
Liechtenstein Dymista Nasenspray
Lithuania Dymista 137 mikrogramai/50 mikrogramu / dozeje nosies purškalas
Luxembourg Dymista Neusspray / Suspension pour pulvérisation nasale / Nasenspray
Malta Dymista Nasal Spray
Norway Dymista nesespray
Poland Dymista
Portugal Dymista Spray nasal
Romania Dymista 137 micrograme / 50 micrograme /doza spray nazal suspensie
Slovakia Dymista nosová aerodisperzia
Slovenia Dymista 137 mikrogramov / 50 mikrogramov na vpih pršilo za nos, suspenzija
Spain Dymista suspensión pulverización nasal
Sweden Dymista Nässpray, suspension (1mg/g; 0.365 mg/g)
United Kingdom (Northern Ireland) Dymista Nasal Spray
Date of the last revision of this leafletFebruary 2019
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS)https://www.aemps.gob.es
- Country of registration
- Average pharmacy price15.61 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DYMISTA 137 micrograms/50 micrograms/application NASAL SPRAY SUSPENSIONDosage form: NASAL PRODUCT, 137 micrograms/50 micrograms/applicationActive substance: fluticasone, combinationsManufacturer: Laboratorios Cinfa S.A.Prescription requiredDosage form: NASAL PRODUCT, 137 micrograms/50 micrograms/actuationActive substance: fluticasone, combinationsManufacturer: Teva B.V.Prescription requiredDosage form: NASAL PRODUCT, 137 micrograms/50 micrograms/applicationActive substance: fluticasone, combinationsManufacturer: Especialidades Farmaceuticas Centrum S.A.Prescription required
Online doctors for DYMISTA 137 micrograms/50 micrograms/application NASAL SPRAY SUSPENSION
Discuss questions about DYMISTA 137 micrograms/50 micrograms/application NASAL SPRAY SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











