DUOTRAV 40 micrograms/ml + 5mg/ml EYE DROPS SOLUTION


How to use DUOTRAV 40 micrograms/ml + 5mg/ml EYE DROPS SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
DuoTrav 40micrograms/ml+5mg/ml eye drops solution
travoprost/timolol
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is DuoTrav and what is it used for
- What you need to know before you use DuoTrav
- How to use DuoTrav
- Possible side effects
- Storing DuoTrav
- Contents of the pack and other information
1. What is DuoTrav and what is it used for
DuoTrav eye drops solution is an association of two active substances (travoprost and timolol). Travoprost is a prostaglandin analogue that acts by increasing the outflow of aqueous fluid from the eye, and thereby reduces the pressure in the eye. Timolol is a beta-blocker that acts by reducing the production of fluid in the inside of the eye. The two substances act together to reduce the pressure in the inside of the eye.
DuoTrav eye drops are used to treat high pressure in the eyes in adults, including elderly patients. This pressure can lead to a disease called glaucoma.
2. What you need to know before you use DuoTrav
Do not use DuoTrav
- if you are allergic to travoprost, prostaglandins, timolol, beta-blockers or any of the other ingredients of this medicine (listed in section 6).
- if you currently have or have a history of respiratory problems such as asthma, severe chronic obstructive pulmonary disease (severe lung disease which may cause wheezing, difficulty in breathing and/or coughing) or other types of respiratory problems.
- if you have severe hay fever.
- if you have a slow heart rate, heart failure or a heart rhythm disorder (irregular heartbeat).
- if the surface of your eye is cloudy.
Consult your doctor if you are in any of these situations.
Warnings and precautions
Consult your doctor before starting to use DuoTrav if you currently have or have a history of
- heart disease (symptoms can include chest pain, shortness of breath or choking), heart failure, low blood pressure.
- heart rhythm disorders such as slow heartbeat.
- respiratory problems, asthma or chronic obstructive pulmonary disease.
- poor circulation (such as Raynaud's disease or Raynaud's syndrome).
- diabetes (as timolol may mask the signs and symptoms of low blood sugar).
- overactive thyroid gland (as timolol may mask the signs and symptoms of thyroid disease).
- myasthenia gravis (a chronic muscle weakness).
- cataract surgery.
- eye inflammation.
If you need to undergo any type of surgery, inform your doctor that you are using DuoTrav, as timolol may affect the effects of some medicines used during anesthesia.
If you suffer any severe allergic reaction (skin rash, redness and itching of the eye) while using DuoTrav, whatever the cause, treatment with adrenaline may not be as effective. Therefore, it is important to inform your doctor that you are using DuoTrav when you receive any other treatment.
DuoTrav may change the color of your iris (the colored part of your eye). This change may be permanent.
DuoTrav may increase the length, thickness, color and/or number of your eyelashes and may cause unusual hair growth on your eyelids.
Travoprost may be absorbed through the skin; therefore pregnant or breast-feeding women should not handle DuoTrav. In case of contact of the medicine with the skin, it should be immediately washed off.
Children
DuoTrav should not be used in children and adolescents under 18 years of age.
Other medicines and DuoTrav
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines, including medicines obtained without a prescription.
DuoTrav may affect or be affected by other medicines you are using, including other eye drops for the treatment of glaucoma. Consult your doctor if you are using or plan to use medicines to lower blood pressure, for the heart including quinidine (used to treat heart conditions and some types of malaria), to treat diabetes or antidepressants fluoxetine or paroxetine.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before using this medicine.
Do not use DuoTrav if you are pregnant unless your doctor considers it necessary. If you can become pregnant, you should use an effective method of birth control while using the medicine.
Do not use DuoTrav if you are breast-feeding. DuoTrav may pass into breast milk.
Driving and using machines
Immediately after applying DuoTrav you may notice that your vision is blurred. In some patients DuoTrav may also cause hallucinations, dizziness, nervousness or fatigue.
Do not drive or use machines until these symptoms have resolved.
DuoTrav contains hydrogenated castor oil and polyoxyl hydrogenated castor oil and propylene glycolwhich may cause skin reactions and irritation.
3. How to use DuoTrav
Follow exactly the instructions of your doctor to use this medicine. If you are unsure, consult your doctor or pharmacist again.
The recommended dose is one drop in the affected eye(s) once daily, in the morning or in the evening. Use it at the same time every day.
DuoTrav should only be used in both eyes if your doctor has told you to do so.
Use DuoTrav only as eye drops.
|
|
|
|
1 | 2 | 3 | 4 |
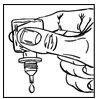
- Immediately before using the bottle for the first time, open the protective foil pouch (figure 1), take out the bottle and write the date you opened it on the space provided on the carton.
- Stand in front of a mirror.
- Wash your hands.
- Remove the cap from the bottle.
- Hold the bottle upside down between your fingers.
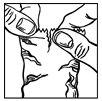
- Tilt your head back. Gently pull the lower eyelid down until a pocket (or pouch) forms between your eye and eyelid.
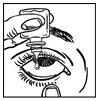
- Bring the bottle tip close to the eye. Looking upwards, gently squeeze the bottle to let one drop fall into the pocket (figure 2).
- Do not touch the eye or the eyelid with the bottle tip, because the eye drops could become contaminated.
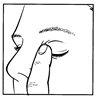
- Gently press the finger into the corner of the eye, by the nose, for about 2 minutes after applying the eye drops (figure 4). This helps to prevent the eye drops from draining down the tear duct and being absorbed into the bloodstream.
- If you need to use DuoTrav in both eyes, repeat the above steps for the other eye.
- Close the bottle tightly after use.
- Use one bottle at a time. Do not open the protective foil pouch until you are ready to use the bottle.
Use DuoTrav for the whole period of time your doctor has told you to use it.
If you use more DuoTrav than you should
If you use more DuoTrav than you should, it may be washed out with warm water. Do not apply extra drops until your next normal dose.
If you forget to use DuoTrav
If you forget to use DuoTrav, continue with the next dose as planned. Do not use a double dose to make up for the forgotten dose. The dose should not exceed one drop per day in the affected eye(s).
If you stop using DuoTrav
If you stop using DuoTrav without consulting your doctor, the pressure in your eye will not be controlled, which could lead to loss of vision.
If you are using another eye drop besides DuoTrav, wait at least 5 minutes between applying DuoTrav and the other eye drops.
If you wear soft contact lenses, do not apply the drops while wearing the lenses. After applying the drops, wait 15 minutes before putting the lenses back in your eyes.
If you have any other questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Generally, unless the effects are severe, you can continue using the eye drops. If these effects bother you, talk to your doctor or pharmacist. Do not stop using DuoTrav without talking to your doctor.
Very common side effects (may affect more than 1 in 10 people)
Eyelid disorders
Eye redness.
Common side effects (may affect up to 1 in 10 people)
Eyelid disorders
Surface inflammation with damage to the surface of the eye, eye pain, blurred vision, abnormal vision, dry eye, eye itching, eye discomfort, signs and symptoms of eye irritation (e.g. burning, stinging).
Uncommon side effects (may affect up to 1 in 100 people)
Eyelid disorders
Surface inflammation of the eye, eyelid inflammation, conjunctival edema, increased length of eyelashes, iris inflammation, eye inflammation, light sensitivity, decreased vision, tired eyes, eye allergy, eye swelling, increased tear production, eyelid redness, change in eyelid color, skin darkening (around the eye).
Other effects
Allergic reaction to the active substance, dizziness, headache, increased or decreased blood pressure, shortness of breath, excessive hair growth, runny nose, skin inflammation with redness and itching, decreased heart rate.
Rare side effects (may affect up to 1 in 1,000 people)
Eyelid disorders
Thinning of the surface of the eye, inflammation of the eyelid glands, broken blood vessel in the eye, crusts on the eyelid, abnormal growth and positioning of eyelashes.
Other effects
Nervousness, irregular heartbeat, hair loss, voice disorders, difficulty breathing, cough, throat irritation, hives, abnormal blood test results for liver, skin discoloration, thirst, fatigue, unusual sensation inside the nose, colored urine, pain in hands and feet.
Frequency not known (cannot be estimated from the available data)
Eyelid disorders
Drooping eyelid (causing the eye to be half-closed), sunken eyes (the eyes appear more deep-set), changes in iris color (the colored part of the eye).
Other effects
Skin rash, heart failure, chest pain, stroke, fainting, depression, asthma, increased heart rate, tingling or numbness, palpitations, swelling in the lower limbs, bad taste.
Additionally:
DuoTrav is an association of two active substances, travoprost and timolol. Like other eye medicines, travoprost and timolol (a beta-blocker) are absorbed into the bloodstream. This may cause side effects similar to those seen with beta-blocker medicines given by mouth or injection. The incidence of side effects after eye administration is lower than for medicines given by mouth or injection.
The following side effects have been observed with the class of beta-blockers used to treat eye conditions or with travoprost alone:
Eyelid disorders
Eyelid inflammation, corneal inflammation, detachment of the layer underneath the retina which contains blood vessels which can cause vision disturbances after filtration surgery, decreased corneal sensitivity, corneal erosion (damage to the outer layer of the eyeball), double vision, eye discharge, eye swelling, eyelid itching, abnormal turning out of the lower eyelid with redness, irritation and increased tear production, blurred vision (sign of cataract), swelling of a part of the eye (uvea), eyelid eczema, visual halos, decreased sensitivity in the eye, pigmentation inside the eye, dilated pupils, change in eyelash color, change in eyelash texture.
Other effects
Ear and labyrinth disorders: dizziness with a feeling of movement, ringing in the ears.
Heart and circulation: slow heart rate, palpitations, edema (fluid accumulation), changes in heart rate or rhythm, congestive heart failure (heart disease with difficulty breathing and swelling of the feet and legs due to fluid accumulation), a type of heart rhythm disorder, heart attack, decreased blood pressure, Raynaud's phenomenon, cold hands and feet, reduced blood flow to the brain.
Respiratory: constriction of airways in the lungs (mainly in patients with pre-existing disease), nasal congestion or runny nose, sneezing (due to allergy), difficulty breathing, nosebleeds, dry nose.
Nervous system and general disorders: difficulty sleeping (insomnia), nightmares, memory loss, hallucinations, loss of strength and energy, anxiety (excessive emotional distress).
Gastrointestinal: altered taste, nausea, indigestion, diarrhea, dry mouth, abdominal pain, vomiting, and constipation.
Allergic reactions: increased allergic symptoms, generalized allergic reactions including swelling under the skin which can occur in areas such as the face and limbs and can obstruct the airways, potentially causing difficulty swallowing or breathing, hives, localized and generalized rash, itching, sudden and severe allergic reaction that can be life-threatening.
Skin: rash with a silvery-white appearance (psoriasiform rash) or worsening of psoriasis, skin peeling, abnormal hair texture, skin inflammation with redness and itching, change in hair color, loss of eyelashes, itching, abnormal hair growth, skin redness.
Muscle: increased signs and symptoms of myasthenia gravis (a chronic muscle weakness), unusual sensations such as tingling, muscle weakness/fatigue, muscle pain not caused by exercise, joint pain.
Kidney and urinary disorders: difficulty and pain when urinating, involuntary urination.
Reproductive: sexual dysfunction, decreased sexual desire.
Metabolism: low blood sugar, increased prostate cancer marker.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing DuoTrav
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the bottle and carton after “EXP”. The expiry date is the last day of the month stated.
Do not store above 30°C.
To avoid infections, you must throw away the bottle 4 weeks after you first opened it and use a new bottle. Write the date you opened it on the space provided on the carton.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Container contents and additional information
DuoTrav composition
- The active ingredients are travoprost and timolol. Each ml of solution contains 40 micrograms of travoprost and 5 mg of timolol (as timolol maleate).
- The other ingredients are Polyquaternium-1, mannitol (E421), propylene glycol (E1520), hydrogenated castor oil and polyoxyl 40, boric acid, sodium chloride, sodium hydroxide or hydrochloric acid (to adjust pH) and purified water.
Very small amounts of sodium hydroxide or hydrochloric acid are added to maintain normal acidity levels (pH levels).
Product appearance and container contents
DuoTrav is a liquid (a colorless and transparent solution) that comes in a 2.5 ml plastic bottle with a screw cap. Each bottle is in a bag.
Packaging of 1, 3 or 6 bottles.
Not all presentations may be marketed.
Marketing authorization holder
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublin 4
Ireland
Manufacturer responsible
Novartis Pharma GmbH
Roonstraße 25
D-90429 Nuremberg
Germany
Novartis Manufacturing NV
Rijksweg 14
2870 Puurs-Sint-Amands
Belgium
Novartis Farmacéutica, S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona
Spain
Siegfried El Masnou, S.A.
Camil Fabra 58
El Masnou
08320
Spain
Novartis Pharma GmbH
Sophie-Germain-Strasse 10
90443 Nürnberg
Germany
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 | Lietuva SIA Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
| Luxembourg/Luxemburg Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 |
Ceská republika Novartis s.r.o. Tel: +420 225 775 111 | Magyarország Novartis Hungária Kft. Tel.: +36 1 457 65 00 |
Danmark Novartis Healthcare A/S Tlf: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Deutschland Novartis Pharma GmbH Tel: +49 911 273 0 | Nederland Novartis Pharma B.V. Tel: +31 88 04 52 111 |
Eesti SIA Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Norge Novartis Norge AS Tlf: +47 23 05 20 00 |
Ελλάδα Novartis (Hellas) A.E.B.E. Τηλ: +30 210 281 17 12 | Österreich Novartis Pharma GmbH Tel: +43 1 86 6570 |
España Novartis Farmacéutica, S.A. Tel: +34 93 306 42 00 | Polska Novartis Poland Sp. z o.o. Tel.: +48 22 375 4888 |
France Novartis Pharma S.A.S. Tél: +33 1 55 47 66 00 | Portugal Novartis Farma - Produtos Farmacêuticos, S.A. Tel: +351 21 000 8600 |
Hrvatska Novartis Hrvatska d.o.o. Tel. +385 1 6274 220 | România Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Ireland Novartis Ireland Limited Tel: +353 1 260 12 55 | Slovenija Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Novartis Slovakia s.r.o. Tel: + 421 2 5542 5439 |
Italia Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Suomi/Finland Novartis Finland Oy Puh/Tel: +358 (0)10 6133 200 |
Κύπρος Novartis Pharma Services Inc. Τηλ: +357 22 690 690 | Sverige Novartis Sverige AB Tel: +46 8 732 32 00 |
Latvija SIA Novartis Baltics Tel: +371 67 887 070 |
Date of last revision of this prospectus
Other sources of information
Detailed information about this medication is available on the European Medicines Agency website: http://www.ema.europa.eu
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DUOTRAV 40 micrograms/ml + 5mg/ml EYE DROPS SOLUTIONDosage form: EYEDROP, 10 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: EYEDROP, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Brill Pharma S.L.Prescription requiredDosage form: EYE DROP, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Laboratorio Stada S.L.Prescription required
Online doctors for DUOTRAV 40 micrograms/ml + 5mg/ml EYE DROPS SOLUTION
Discuss questions about DUOTRAV 40 micrograms/ml + 5mg/ml EYE DROPS SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions