
DROPIZOL 10 mg/ml ORAL DROPS IN SOLUTION

How to use DROPIZOL 10 mg/ml ORAL DROPS IN SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Dropizol 10 mg/ml Oral Drops Solution
Morphine in Opium Tincture
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Dropizol and what is it used for
- What you need to know before you take Dropizol
- How to take Dropizol
- Possible side effects
- Storage of Dropizol
- Contents of the pack and other information
1. What is Dropizol and what is it used for
Dropizol is a herbal-based medicine that contains morphine.
Dropizol belongs to a group of medicines called antipropulsives and is used in adults to treat the symptoms of acute diarrhea when the use of other antidiarrheal treatments has not had the sufficient effect.
Dropizol works by inhibiting intestinal motility.
2. What you need to know before you take Dropizol
Do not take Dropizol:
- if you are allergic to opium or morphine or any of the other ingredients of this medicine (listed in section 6).
- if you are addicted to opioids.
- if you have glaucoma (increased intraocular pressure).
- if you have severe liver or kidney disease.
- if you have symptoms of alcohol withdrawal (delirium tremens).
- if you have a severe head injury.
- if you are at risk of paralytic ileus (obstruction of the intestine due to paralysis of the intestinal muscles).
- if you have acute asthma.
- if you have a chronic lung disease that makes breathing difficult (COPD).
- if you have respiratory problems due to severe respiratory depression. Your doctor will inform you if you have any of these conditions. Symptoms may include difficulty breathing, coughing, or slower or weaker breathing than expected.
- if you have heart failure secondary to lung disease (cor pulmonale).
- if you are breastfeeding.
Warnings and precautions
Consult your doctor, pharmacist, or nurse before taking Dropizol:
- if you are an elderly patient, as this group of patients may react differently to this medicine. The dose may need to be adjusted.
- if you have chronic kidney or liver disease. The dose may need to be adjusted.
- if you are addicted to sedatives or alcohol.
- if you have a gallbladder disease or gallstones.
- if you have a head injury or increased intracranial pressure.
- if you have a reduced level of consciousness.
- if you are taking medicines for depression (moclobemide or other MAO inhibitors) or have stopped taking this medicine in the last 2 weeks.
- if you have adrenal insufficiency.
- if you have hypothyroidism, the dose may need to be adjusted.
- Hypotension, accompanied by a reduced blood volume.
- Pancreatitis.
- if you have prostate hyperplasia (enlargement of the prostate) or disorders that predispose to urinary retention.
- if you have an intestinal infection or inflammation, as the inhibition of intestinal peristalsis could increase the risk of toxin absorption and the development of colon hypertrophy and intestinal perforation.
- if you have epilepsy.
- if you are taking other medicines for diarrhea.
- if you have convulsions.
- if you have a stomach or duodenal hemorrhage.
- if you are taking medicines to treat blood pressure.
If you experience difficulty urinating, you should contact a healthcare professional.
Dropizol is not recommended before surgery or in the 24 hours following surgery, due to the risk of paralytic ileus, whose symptoms are nausea and vomiting.
Risk of dependence and tolerance with the use of the medicine.
Tolerance, dependence, and addiction.
This medicine contains morphine, which is an opioid medicine. Repeated use of opioids can make the drug less effective (one gets used to it, which is known as tolerance). Repeated use of Dropizol can also cause dependence, abuse, and addiction, which can lead to a potentially fatal overdose. The risk of these side effects may increase with a higher dose and longer duration of use.
Dependence or addiction can make you feel like you no longer have control over the amount of medicine you need to take or how often you need to take it.
The risk of becoming dependent or addicted varies from person to person. You may have a higher risk of becoming dependent or addicted to Dropizol if:
- You or someone in your family has ever abused or been dependent on alcohol, prescription drugs, or illegal drugs ("addiction").
- You are a smoker.
- You have ever had problems with your mood (depression, anxiety, or personality disorder) or have been treated by a psychiatrist for other mental illnesses.
If you notice any of the following signs while taking Dropizol, it could be a sign that you have become dependent or addicted:
- You need to take the medicine for a longer period than recommended by your doctor.
- You need to take more doses than recommended.
- You are using the medicine for reasons other than those prescribed, for example, "to calm down" or "to help you sleep".
- You have made repeated unsuccessful attempts to stop or control the use of the medicine.
- When you stop taking the medicine, you feel unwell and feel better when you take the medicine again ("withdrawal effects").
If you notice any of these signs, talk to your doctor to discuss the best course of treatment for you, including when it is appropriate to stop and how to do it safely (see section 3, If you stop taking Dropizol).
Children and adolescents
Dropizol should not be used in children and adolescents under 18 years of age.
Acute generalized exanthematous pustulosis (AGEP) has been reported in association with Dropizol treatment. Symptoms usually appear within the first 10 days of treatment. Inform your doctor if you have ever developed a severe skin rash, skin peeling, blisters, and/or mouth sores after taking Dropizol or other opioids. Stop using Dropizol and seek medical attention immediately if you notice any of the following symptoms: blisters, widespread scaly skin, or pus-filled spots along with fever.
Sleep-related breathing disorders
Dropizol may cause sleep-related breathing disorders, such as sleep apnea (pauses in breathing during sleep) and sleep-related hypoxemia (low oxygen level in the blood). Symptoms may include breathing pauses during sleep, nighttime awakenings due to lack of air, difficulty maintaining sleep, or excessive daytime sleepiness. If you or someone else observes these symptoms, contact your doctor. Your doctor may consider a dose reduction.
Contact your doctor if you experience severe upper abdominal pain that may radiate to the back, nausea, vomiting, or fever, as these may be symptoms associated with pancreatitis and biliary tract disease.
Using Dropizol with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
It is especially important that you tell your doctor or pharmacist if you are taking any of the following medicines:
- Medicines that enhance the reduction of the level of consciousness and respiratory difficulties observed with the use of Dropizol, such as:
- Alcohol
- Sedatives (e.g., zolpidem) and general anesthetics (e.g., barbiturics)
- Medicines for treating depression (tricyclic antidepressants) or Parkinson's disease (MAO inhibitors, e.g., safinamide)
- Antipsychotic medicines with sedative action (e.g., phenothiazines)
- Medicines for treating epilepsy and pain due to nerve problems (gabapentin or pregabalin)
- Medicines for relieving nausea and vomiting (e.g., bromoprida, meclocina, metoclopramida)
- Medicines for relieving allergies (antihistamines, e.g., carbinoxamine, doxylamine)
- Other opioid analgesics (e.g., alfentanil, butorphanol, fentanyl, hydrocodone, hydromorphone, levorphanol, meperidine, methadone, oxycodone, oxymorphone, remifentanil, sufentanil, tapentadol, tramadol)
The simultaneous administration of morphine may increase the effects of medicines that reduce blood pressure or other medicines that have an effect on blood pressure.
Using Dropizol with food, drinks, and alcohol
Dropizol can be taken with food and drinks. Dropizol contains alcohol, so caution should be exercised if alcohol is consumed.
See the section below Dropizol contains ethanol.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Pregnancy:
Dropizol should not be used during pregnancy unless your doctor has told you to do so. Caution should be exercised when using this medicine in pregnant women. Dropizol should not be used near the delivery date due to the risk of withdrawal symptoms in the newborn.
Breastfeeding:
Dropizol should not be used during breastfeeding.
Fertility
It is not known whether morphine may negatively affect fertility. Men and women of childbearing age should use effective contraceptive methods during treatment with Dropizol.
Driving and using machines
Dropizol contains morphine and ethanol and may cause drowsiness and significantly affect your ability to drive or use machines.
Do not drive after taking medicines until you know how they affect you.
Dropizol contains ethanol
This medicine contains 33% ethanol by volume (alcohol); which corresponds to an amount of 260 mg per dose, equivalent to 6.6 ml of beer or 2.8 ml of wine per dose. This medicine is harmful to people with alcoholism. The alcohol content should be taken into account in pregnant or breastfeeding women, children, and high-risk groups, such as patients with liver disease or epilepsy.
3. How to take Dropizol
Follow exactly the administration instructions of this medicine given by your doctor. If you are in doubt, consult your doctor or pharmacist again.
The recommended dose in adults is:
Adults: 5-10 drops 2-3 times a day.
The single dose should not exceed 1 ml, and the total daily dose should not exceed 6 ml.
Elderly patients: the initial dose should be reduced.
Liver failure: Dropizol should not be used, or the dose should be reduced. See the section Do not take Dropizol and Warnings and precautionsin section 2.
Kidney failure: Dropizol should not be used, or the dose should be reduced. See the section Do not take Dropizol and Warnings and precautionsin section 2.
Each milliliter contains approximately 20 drops.
Method of administration:
Oral route.
Dropizol can be used undiluted or mixed with a glass of water. After mixing with water, it should be taken immediately. If Dropizol is used undiluted, the correct dose can be administered with a spoon.
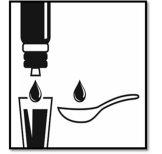
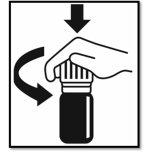
- Open the child-resistant closure: 2. Hold the bottle in a vertical position
Press down and turn. and drop onto a spoon or a glass.
Use in children and adolescents
Dropizol should not be used in children and adolescents under 18 years of age.
If you take more Dropizol than you should
If you take too much Dropizol, you may experience constricted pupils, reduced heart rate, low blood pressure, pulmonary edema, breathing difficulties, and reduced level of consciousness, which could lead to coma.
Call your doctor if you have taken more Dropizol than prescribed or if you take it as indicated in the package leaflet and feel uncomfortable.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 915 620 420, indicating the medicine and the amount ingested.
If you forget to take Dropizol
You should take the missed dose as soon as possible, unless it is almost time for the next dose. Do not take a double dose to make up for forgotten doses.
If you stop taking Dropizol
Keep taking the medicine for the time your doctor has told you.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them. Stop using Dropizol and seek immediate medical attention if you notice any of the following symptoms.
Severe Adverse Effects
Frequent(may affect up to 1 in 10 people):
Difficulty urinating
Infrequent(may affect up to 1 in 100 people):
Shortness of breath, fatigue, anxiety, bluish discoloration of the lips and fingers and toes, headaches, confusion, convulsions, and swelling of the legs and feet (respiratory depression).
Cardiac arrhythmia (rapid or slow heart rate)
Other Adverse Effects
Very Frequent(may affect more than 1 in 10 people):
Drowsiness and constipation, dry mouth
Frequent(may affect up to 1 in 10 people):
Dizziness, headache, pupil constriction, nausea and vomiting, loss of appetite, indigestion or discomfort, alterations in taste and smell, hives, sweating, bronchospasm, decreased cough, asthenia
Infrequent(may affect up to 1 in 100 people):
Facial flushing, itching, spasms in the lower urinary tract, alteration in liver function tests
Rare(may affect up to 1 in 1,000 people):
Increased concentration of liver enzymes (observed in a blood test) and pancreatitis, pain due to the presence of kidney stones (renal colic) or gallstones (biliary colic), withdrawal symptoms, orthostatic hypotension (a type of hypotension that occurs when standing up, having been seated or lying down)
Very Rare(may affect up to 1 in 10,000 people):
Respiratory difficulty, muscle cramps, convulsions, burning and itching, increased sensitivity to pain, blurred vision, double vision, involuntary eye movement, a disease in which the intestine does not function properly (ileus), abdominal pain, rash, swelling of the hands, ankles, or feet, general malaise, chills, syndrome of inappropriate antidiuretic hormone secretion (SIADH) (symptoms: nausea, general malaise, headache, exhaustion, and in severe cases, may progress to convulsions and coma), amenorrhea.
Unknown Frequency(cannot be estimated from available data):
Adrenal insufficiency (fatigue, weight loss, fainting, hypoglycemia, nausea, diarrhea, vomiting, and abdominal pain), euphoria (strong feeling of well-being, happiness, and excitement), uncontrolled muscle movements, addiction, dysphoric state (sadness, lack of energy), restlessness, decreased libido or potency, hallucinations, vertigo, and fever. Severe skin reaction with blisters, scaly skin, pus-filled spots, and fever. This could be a condition called acute generalized exanthematous pustulosis (AGEP). Sleep apnea (pauses in breathing during sleep). Symptoms associated with pancreatitis and biliary tract inflammation, e.g., severe pain in the upper abdomen that may radiate to the back, nausea, vomiting, or fever.
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines www.notificaRAM.es.
By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Dropizol
Keep this medicine out of sight and reach of children.
Do not use this medicine after the expiration date shown on the box and bottle. The expiration date is the last day of the month indicated.
Do not refrigerate or freeze.
The bottle must be used within 4 weeks after the first opening.
Medicines should not be thrown away through wastewater or household waste. Deposit the packaging and medicines you no longer need at the SIGRE point in the pharmacy or any other system for collecting medicinal waste. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Composition of Dropizol
- The active ingredient is: opium tincture
Each milliliter of oral solution contains 1 ml of Papaver somniferum(L.), Succus siccus(pure opium) tincture, which is equivalent to 10 mg of morphine.
Each drop contains 50 mg of opium tincture, which is equivalent to 0.5 mg (10 mg/ml) of anhydrous morphine.
Each milliliter contains 20 drops.
- Extraction solvent: 33% ethanol (v/v)
- Other components are:
- 96% ethanol (v/v)
- Purified water
Appearance of the Product and Package Contents
Dropizol is a dark brown-red liquid. It is available in a brown glass bottle with a dropper and child-resistant closure.
Presentation: 1 × 10 ml, 2 × 10 ml, 3 × 10 ml, 4 × 10 ml, 5 × 10 ml, and 10 × 10 ml.
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Pharmanovia A/S
Ørestads Boulevard 108, 5
DK-2300 Copenhagen S
Denmark
Email: [email protected]
Manufacturer
Lomapharm GmbH
Langes Feld 5
D-31860 Emmerthal
Germany
Atnahs Pharma Denmark ApS
Copenhagen Towers,
Ørestads Boulevard 108, 5.tv,
DK-2300 Copenhagen S,
Denmark
You can request more information about this medicine by contacting the local representative of the Marketing Authorization Holder:
Denmark Pharmanovia A/S Ørestads Boulevard 108 DK-2300 Copenhagen S Denmark | Norway Pharmanovia A/S Ørestads Boulevard 108 DK-2300 Copenhagen S Denmark |
Iceland Pharmanovia A/S Ørestads Boulevard 108 DK-2300 Copenhagen S Denmark | Finland Pharmanovia A/S Ørestads Boulevard 108 DK-2300 Copenhagen S Denmark |
Sweden Pharmanovia A/S Ørestads Boulevard 108 DK-2300 Copenhagen S Denmark | UK Pharmanovia A/S Ørestads Boulevard 108 DK-2300 Copenhagen S Denmark |
Austria Pharmanovia A/S Ørestads Boulevard 108 DK-2300 Copenhagen S Denmark | Belgium Pharmanovia A/S Ørestads Boulevard 108 DK-2300 Copenhagen S Denmark |
Czech Republic Pharmanovia A/S Ørestads Boulevard 108 DK-2300 Copenhagen S Denmark | Germany Pharmanovia A/S Ørestads Boulevard 108 DK-2300 Copenhagen S Denmark |
Spain Pharmanovia A/S Ørestads Boulevard 108 DK-2300 Copenhagen S Denmark | France Pharmanovia A/S Ørestads Boulevard 108 DK-2300 Copenhagen S Denmark |
Hungary Pharmanovia A/S Ørestads Boulevard 108 DK-2300 Copenhagen S Denmark | Ireland Pharmanovia A/S Ørestads Boulevard 108 DK-2300 Copenhagen S Denmark |
Italy Pharmanovia A/S Ørestads Boulevard 108 DK-2300 Copenhagen S Denmark | Luxembourg Pharmanovia A/S Ørestads Boulevard 108 DK-2300 Copenhagen S Denmark |
Netherlands Pharmanovia A/S Ørestads Boulevard 108 DK-2300 Copenhagen S Denmark | Portugal Pharmanovia A/S Ørestads Boulevard 108 DK-2300 Copenhagen S Denmark |
Romania Pharmanovia A/S Ørestads Boulevard 108 DK-2300 Copenhagen S Denmark | Slovak Republic Pharmanovia A/S Ørestads Boulevard 108 DK-2300 Copenhagen S Denmark |
This medicine is authorized in the Member States of the European Economic Area under the following names:
Denmark: Dropizol
Iceland: Dropizol
Finland: Dropizol
Norway: Dropizol
Sweden: Dropizol
UK: Dropizol
Austria: Dropizol
Belgium: Dropizole
Czech Republic: Dropizol
Germany: Dropizol
Spain: Dropizol
France: Dropizal
Hungary: Dropizol
Ireland: Dropizol
Italy: Dropizole
Luxembourg: Dropizol
Netherlands: Dropizol
Portugal: Dropizale
Romania: Dropizol
Slovak Republic: Dropizol
Date of the last revision of this prospectus:June 2024.
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DROPIZOL 10 mg/ml ORAL DROPS IN SOLUTIONDosage form: TABLET, 2 mgActive substance: loperamideManufacturer: Uxa Farma S.A.Prescription not requiredDosage form: CAPSULE, 2 mg loperamide hydrochlorideActive substance: loperamideManufacturer: Laboratorios Cinfa S.A.Prescription not requiredDosage form: ORALLY DISINTEGRATING TABLET/LIOTAB, 2 mgActive substance: loperamideManufacturer: Laboratorios Cinfa S.A.Prescription not required
Online doctors for DROPIZOL 10 mg/ml ORAL DROPS IN SOLUTION
Discuss questions about DROPIZOL 10 mg/ml ORAL DROPS IN SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











