
DEXAMETHASONE POS 1 mg/ml EYE DROPS SOLUTION


How to use DEXAMETHASONE POS 1 mg/ml EYE DROPS SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Dexamethasone POS 1 mg/ml Eye Drops Solution
Dexamethasone metasulfobenzoate sodium
Read this package leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What Dexamethasone POS is and what it is used for
- What you need to know before you use Dexamethasone POS
- How to use Dexamethasone POS
- Possible side effects
- Storage of Dexamethasone POS
- Contents of the pack and further information
1. What Dexamethasone POS is and what it is used for
Dexamethasone POS is a glucocorticoid for local treatment of the eye.
Dexamethasone POS is used for the treatment of non-infectious inflammatory conditions of the eyes that respond to corticosteroids, such as eye allergies, inflammatory disorders of the conjunctiva, corneal inflammation, and inflammation of the anterior segment of the eye; postoperative irritations.
2. What you need to know before you use Dexamethasone POS
Do not useDexamethasone POS
- if you are allergic to dexamethasone metasulfobenzoate sodium or to any of the other ingredients of this medicine (listed in section 6),
- in case of acute simple herpes (dendritic keratitis) and other viral eye infections,
- in case of bacterial and/or fungal eye infections without appropriate antibiotic therapy,
- in case of corneal ulcers or injuries,
- in case of narrow-angle glaucoma and open-angle glaucoma,
- in case of weakened immune system caused by diseases or medications.
Warnings and precautions
Consult your doctor or pharmacist before you start using Dexamethasone POS.
In case you have previously suffered from a simple herpes infection or eye surgery, this medicine should only be applied under strict medical control.
When using this medicine for 10 days or more, your doctor should regularly check your intraocular pressure and cornea.
In order to avoid possible systemic absorption, especially in small children, you should close the tear duct with your finger for 2 to 3 minutes after applying this medicine.
Talk to your doctor if you experience swelling and weight gain around the trunk and face, as these are usually the first signs of a syndrome called Cushing's Syndrome. Suppression of adrenal gland function may develop after prolonged or intensive treatment with this medicine. Talk to your doctor before stopping treatment on your own. These risks are especially important in children and patients treated with a medicine called ritonavir or cobicistat.
Contact your doctor if you experience blurred vision or other visual disturbances.
Using Dexamethasone POS with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Concomitant use of this medicine with some drugs that affect the nervous system, such as atropine, may cause an additional increase in intraocular pressure.
Tell your doctor if you are taking ritonavir or cobicistat, as this may increase the amount of dexamethasone in your blood.
Warning:
When using other topical eye medicines concomitantly, there should be an interval of 15 minutes between the applications of the different products. Ointments should always be administered last.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
There are no validated data on the use of this medicine during pregnancy. Since glucocorticoids can cause malformations in animal studies, this medicine should only be used during pregnancy if strictly indicated.
It is not known whether topical application of this medicine can lead to detectable levels of dexamethasone in breast milk through systemic absorption. Systemic administration of glucocorticoids is known to pass into breast milk and may cause growth disorders in the infant. Therefore, this medicine should only be used during breastfeeding if strictly indicated.
Driving and using machines
Do not drive immediately after applying this medicine, as it reduces vision for a short period of time and thus also reduces reaction time when driving. You should also not operate electrical tools or machinery temporarily.
Dexamethasone POScontains
This medicine contains dexamethasone metasulfobenzoate sodium as the active ingredient, which may cause allergic reactions. Inform your doctor if you have any allergies.
Remove your contact lenses before applying this medicine and wait at least 20 minutes before putting them back in.
3. How to use Dexamethasone POS
Follow the instructions for administration of this medicine exactly as indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
If your doctor does not indicate otherwise, the recommended dose is:
3-5 times a day (in acute cases more frequently), 1 drop in the conjunctival sac.
To ensure therapeutic effect, treatment should not be interrupted before the scheduled time. Your doctor will determine the duration of treatment. If treatment lasts more than 2 weeks, your eyes should be regularly examined by your doctor. In any case, long-term treatment without medical supervision should be avoided.
How to use Dexamethasone POS
Wash your hands.

Step 1: Take a clean cloth and gently wipe away the tear fluid from the lower part of the eye.

Step 2: Remove the cap before each application. Before the first application of Dexamethasone POS, hold the container vertically with the tip pointing down and press the base until the first drop appears on the nozzle. The container is now ready for use.
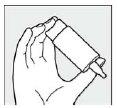
Step 3: Hold the container with the dropper pointing down, so that your thumb presses the shoulder of the container and the rest of your fingers are on the base.
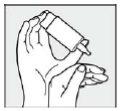
Step 4: Rest the hand holding the COMOD container on the free hand, as shown.
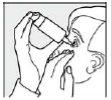
Step 5: Tilt your head back slightly, gently pull down the lower eyelid, and press the base of the container firmly and quickly. This activates the mechanism for the release of one drop. Thanks to the special valve technology of the COMOD system, the size and speed of the drop are identical, regardless of the pressure applied. During application, avoid contact between the tip of the container and the eye or skin. Slowly close your eyes so that the liquid is evenly distributed over the surface of the eye.

Step 6: After applying Dexamethasone POS, blink several times to distribute the drop over the entire ocular surface. Then remove any excess medication.
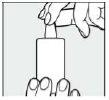
Step 7:
After application, immediately replace the cap. Check that the tip of the dropper is dry.
Repeat this procedure in the other eye.
Distribute the applications of Dexamethasone POS evenly throughout the day.
If you use moreDexamethasone POSthan you should
To date, no symptoms of overdose due to excessive use in the eye have been reported. In case of overdose, rinse the eye with water. If accidental oral ingestion of the medicine occurs (e.g., in children), drink water (½ glass) to dilute it. Normally, no additional measures are required. However, in the case of small children, you should consult a doctor.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to useDexamethasone POS
Do not use a double dose, but apply the same amount and frequency as described above or as prescribed by your doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
For the assessment of side effects, the following frequencies are defined: Very common: more than 1 in 10 patients treated Common: between 1 in 100 and 1 in 10 patients treated Uncommon: between 1 in 1000 and 1 in 100 patients treated Rare: between 1 in 10,000 and 1 in 1,000 patients treated Very rare: less than 1 in 10,000 patients treated Frequency not known: cannot be estimated from the available data
The frequency of the following side effects is not known.
Hormonal problems: increased body hair (especially in women), weakness and muscle wasting, purple striae on the skin, increased blood pressure, irregular or absent periods, changes in protein and calcium levels, stunted growth in children and adolescents, swelling and weight gain around the body and face (called "Cushing's Syndrome") (see section 2).
Ocular disordersLong-term treatment may cause an increase in intraocular pressure in patients with a particular predisposition. Therefore, it is advisable to regularly check intraocular pressure. Additionally, during long-term treatment, irreversible opacity of the lens (cataract) may occur.
A pre-existing infection may be masked or worsen. At the same time, the possibility of a secondary infection should be considered during long-term treatment, especially if signs of chronic infection persist despite treatment. In cases of diseases that cause thinning of the cornea, treatment may lead to perforation.
Cases of allergic reactions such as skin irritation with redness and itching in the eye (contact dermatitis, dermato-conjunctivitis, and eyelid eczema) have been reported. Eye drops containing dexamethasone slow down wound healing. If eye drops containing cortisone are used after cataract surgery, wound healing may be delayed. Long-term treatment with this medicine may also delay wound healing.
After application of eye drops containing glucocorticoids, hypersensitivity reactions with inflammatory symptoms, corneal ulcers, pupil dilation, accommodation disorders (focusing), and ptosis (drooping of the upper eyelid) may occur. Additionally, temporary burning and itching in the eye, as well as eye irritation, blurred vision, foreign body sensation, and allergic reactions may occur.
Reporting of side effects:
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this package leaflet. You can also report side effects directly through the Spanish Medicines and Health Products Agency (AEMPS) website: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Dexamethasone POS
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the container after EXP. The expiry date is the last day of the month shown.
Do not store above 25°C.
Information on the shelf life after opening:
Dexamethasone POS can be used for up to 4 weeks after first opening.
Medicines should not be disposed of via wastewater or household waste. Dispose of the container and any unused medicine in the pharmacy's SIGRE collection point. If in doubt, ask your pharmacist how to dispose of the container and any unused medicine. This will help protect the environment.
6. Contents of the pack and further information
Composition of Dexamethasone POS
- The active ingredient is dexamethasone metasulfobenzoate sodium 1.0 mg/ml
- The other ingredients are hypromellose, glycerol, and water for injections.
Appearance of the product and contents of the pack
Dexamethasone POS is presented in a multidose container with an airless pump system containing 5 ml of clear and colorless eye drops solution. Each carton contains one container.
The filling of the container is carried out under regular control so that a filling with a smaller amount of solution can be excluded.
Marketing authorization holder and manufacturer
Marketing authorization holder
BRILL PHARMA, S.L.
C/ Munner, 8
08022, Barcelona
Spain
Manufacturer
URSAPHARM Arzneimittel GmbH
Industriestraße 35,
66129 Saarbrücken,
Germany
Date of the last revision of this package leaflet:October 2019
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DEXAMETHASONE POS 1 mg/ml EYE DROPS SOLUTIONDosage form: EYEDROP, 1 mg/mlActive substance: dexamethasoneManufacturer: Pharmaselect International Beteiligungs GmbhPrescription requiredDosage form: EYE DROP, 0.1 %Active substance: dexamethasoneManufacturer: Fidia Farmaceutici S.P.A.Prescription requiredDosage form: EYEDROP, 1mg/mlActive substance: dexamethasoneManufacturer: Laboratoires TheaPrescription required
Online doctors for DEXAMETHASONE POS 1 mg/ml EYE DROPS SOLUTION
Discuss questions about DEXAMETHASONE POS 1 mg/ml EYE DROPS SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















