
DACEPTON 10 mg/ml Injectable Solution in Cartridge

Ask a doctor about a prescription for DACEPTON 10 mg/ml Injectable Solution in Cartridge

How to use DACEPTON 10 mg/ml Injectable Solution in Cartridge
Introduction
Package Leaflet: Information for the User
Dacepton 10 mg/ml solution for injection in cartridge EFG
Apomorphine hydrochloride hemihydrate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
The name of your medicine is Dacepton 10 mg/ml solution for injection in cartridge EFG, and it will be referred to as Dacepton 10 mg/ml in cartridge throughout this leaflet.
Contents of the pack
- What Dacepton 10 mg/ml in cartridge is and what it is used for
- What you need to know before you use Dacepton 10 mg/ml in cartridge
- How to use Dacepton 10 mg/ml in cartridge
- Possible side effects
- Storage of Dacepton 10 mg/ml in cartridge
- Contents of the pack and further information
1. What Dacepton 10 mg/ml in cartridge is and what it is used for
Dacepton contains a solution for injection of apomorphine. It is injected under the skin (subcutaneously) using only the D-mine Pen, which is designed for this purpose. The active substance in Dacepton is apomorphine hydrochloride hemihydrate. Each milliliter of solution contains 10 mg of apomorphine hydrochloride hemihydrate.
Apomorphine hydrochloride hemihydrate belongs to a group of medicines known as dopamine agonists. Dacepton is used to treat Parkinson's disease. Apomorphine helps to reduce the time spent in the 'off' or immobile state in people previously treated for Parkinson's disease with levodopa (another treatment for Parkinson's disease) and/or with other dopamine agonists.
Your doctor or nurse will help you recognize the signs that indicate when you should use your medicine.
Despite its name, apomorphine does not contain morphine.
2. What you need to know before you use Dacepton 10 mg/ml in cartridge
Do not use Dacepton 10 mg/ml in cartridge:
- if you are allergic to apomorphine or any of the other ingredients of this medicine (listed in section 6).
- if you are under 18 years of age.
- if you have breathing difficulties.
- if you suffer from dementia or Alzheimer's disease.
- if you have a mental illness with symptoms such as hallucinations, delirium, confused thoughts, loss of contact with reality.
- if you have liver problems.
- if you suffer from severe dyskinesia (involuntary movements) or severe dystonia (inability to move) despite taking levodopa.
- if you know that you or a family member have a heart condition known as 'long QT syndrome'. Inform your doctor.
- if you are taking ondansetron (a medicine used to treat nausea and vomiting).
Warnings and precautions
Before using Dacepton, your doctor will perform an electrocardiogram (ECG), and ask you for a list of all other medicines you are taking. This ECG will be repeated in the first few days of treatment, and at any time your doctor considers it necessary. Your doctor will also ask you about other illnesses you may have, especially those related to the heart. Some of the questions and additional tests may be repeated at each medical visit. If you experience symptoms that may be related to your heart, such as palpitations, fainting, or dizziness, you should inform your doctor immediately. If you have diarrhea or start using a new medicine, you should also inform your doctor.
Consult your doctor, pharmacist, or nurse before starting to use Dacepton:
- if you have kidney problems
- if you have lung problems
- if you have heart problems
- if you have low blood pressure or feel weak or dizzy when standing up
- if you are taking medicines to treat high blood pressure
- if you have nausea or feel dizzy
- if Parkinson's disease causes you any mental problems such as hallucinations and confusion.
- if you are elderly or weak
Tell your doctor if you, your family, or your caregiver notice that you have impulses or irresistible desires to behave in a way that is unusual for you and that you cannot resist the impulse, desire, or temptation to perform certain activities that could harm you or others. These are called impulse control disorders and may include behaviors such as addictive gambling, overeating, or spending money excessively, an abnormally high sexual desire, or an increase in sexual thoughts or feelings. Your doctor may need to adjust or interrupt the dose.
Some patients develop symptoms of addiction that lead to a compulsive desire to consume high doses of Dacepton and other medicines used to treat Parkinson's disease.
Children and adolescents
Dacepton should not be used in children and adolescents under 18 years of age.
Using Dacepton 10 mg/ml in cartridge with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Consult your doctor or pharmacist before taking your medicine
If you are taking medicines that are known to affect the way your heart beats. This includes medicines used to treat heart rhythm problems (such as quinidine and amiodarone), for depression (including tricyclic antidepressants, such as amitriptyline and imipramine), and for bacterial infections (macrolide antibiotics such as erythromycin, azithromycin, and clarithromycin), and domperidone.
If you are taking ondansetron (a medicine used to treat nausea and vomiting), as this may result in a severe drop in blood pressure and loss of consciousness.
If you use Dacepton with other medicines, the effect of those medicines may be altered.
This is especially true for:
- Medicines such as clozapine for treating certain mental disorders.
- Medicines for reducing high blood pressure.
- Other medicines for Parkinson's disease.
Your doctor will tell you if you need to adjust the dose of apomorphine or any other medicine you are using.
If you are taking levodopa (another medicine for Parkinson's disease) in addition to apomorphine, your doctor should perform periodic blood tests.
Using Dacepton 10 mg/ml in cartridge with food and drink
Food and drink do not affect the way Dacepton works.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Dacepton should not be used during pregnancy unless it is strictly necessary.
It is not known if Dacepton passes into breast milk. Consult your doctor if you are breastfeeding or planning to breastfeed. Your doctor will explain whether you should continue or stop breastfeeding or continue or stop this medicine.
Driving and using machines
Dacepton can cause drowsiness and a strong desire to sleep. Do not drive or operate tools or machines if Dacepton has this effect on you.
Dacepton 10 mg/ml in cartridge contains sodium metabisulfite
Sodium metabisulfite can rarely cause severe allergic reactions with symptoms such as rash or itching of the skin, difficulty breathing, swelling of the eyelids, face, or lips, inflammation or redness of the tongue. If you experience these side effects, go immediately to the emergency department of the nearest hospital.
This medicine contains less than 23 mg (1 mmol) of sodium per 10 ml; i.e., it is essentially 'sodium-free'.
3. How to use Dacepton 10 mg/ml in a cartridge
Follow the administration instructions for this medication exactly as indicated by your doctor. In case of doubt, consult your doctor or pharmacist again.
Before using Dacepton, your doctor will check that you tolerate this medication and an antiemetic medication that you must take simultaneously.
You should take domperidone for at least 2 days before starting Dacepton 10 mg/ml to avoid nausea or feeling dizzy.
DO NOT use Dacepton 10 mg/ml in a cartridge if
- the solution has turned green.
- the solution is cloudy or particles can be seen in it.
How much to use
Both the amount of Dacepton you should use and the number of daily injections needed will depend on your personal needs. Your doctor will discuss this with you and tell you the amount of medication to be administered and how often.
The most suitable amount for you will be determined during your visit to the specialist.
- The usual daily dose ranges from 3 mg to 30 mg.
- You may need up to 100 mg per day.
- Normally, you will need between 1 and 10 injections per day.
- Each individual injection should not contain more than 10 mg.
The D-mine Pen pen needed for the application of Dacepton 10 mg/ml in a cartridge is not suitable for patients who need doses higher than 6 mg per injection.
For these patients, other products should be used.
It is not necessary to dilute Dacepton before use. Additionally, it should not be mixed with any other medication.
- Your doctor will indicate the dose of Dacepton you should use and how often. They will also inform you about how to change the dose of Dacepton if necessary. Do not change your dose of Dacepton or use it more frequently unless your doctor tells you to.
- Your doctor will provide you and your caregivers with detailed instructions on the preparation and administration of doses, paying special attention to the correct use of the required administration pen.
Before using Dacepton 10 mg/ml in a cartridge
Note: This packaging DOES NOT include the pen or the pen needles.
Dacepton 10 mg/ml in a cartridge is designed to be administered only with the "D-mine Pen" pen intended for this purpose and disposable needles, as specified in the Pen Instructions for Use.
Description of the pen
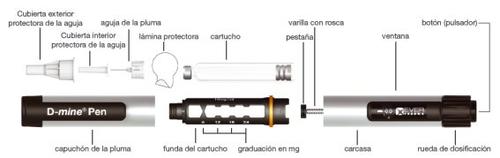
- Always use a new needle for each injection to prevent contamination.
- Do not share needles or pens.
Before using Dacepton 10 mg/ml in a cartridge, examine your pen and the pen manual to familiarize yourself with its correct handling.
- If your pen is damaged or not working correctly (due to mechanical defects), read the Pen Instructions for Use.
Where and how to inject Dacepton 10 mg/ml in a cartridge
- First, wash your hands.
- Before using the pen, you will need surgical wipes and a needle with its protective cone.
- Follow the instructions in your pen manual.
Preparing the pen / changing the cartridge
Remove the pen from its packaging and remove the cap.

Remove the cartridge holder by turning it clockwise.
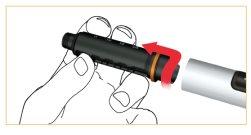
Insert the new cartridge into the holder.
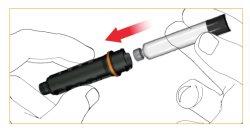
Push the threaded rod all the way to the bottom. This is best done with the tip of your finger.
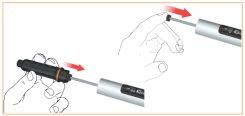
Put the cartridge holder into the body and turn it counterclockwise to secure it.

Adjusting the cartridge needle
Follow the instructions for using the pen needle. Remove the protective film.
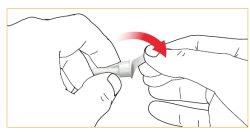
Attach/turn the pen needle to the cartridge holder.
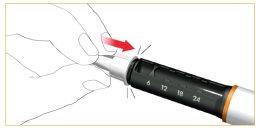
Remove the outer protective cover of the needle. Keep it to safely dispose of the cartridge needle after use.
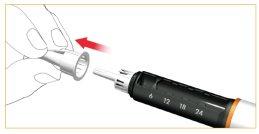
Remove and discard the inner protective cover of the needle.

Priming / operation control
All air in the cartridge must be eliminated before use. Mark the dose forward by turning the dosing wheel. Check the marked dose by looking at the window vertically from above, and not at an angle, so that the "?" symbol is clearly visible. This is called "priming" and is important to ensure that you receive a complete dose when using the pen.

To control operation, hold the pen upright and gently tap the cartridge holder so that the air rises upwards.
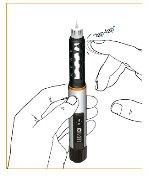
Press the button.

A few drops will come out of the pen needle tip. If no drops come out, repeat this step.

Dose adjustment
Mark your dose by turning the dosing wheel clockwise. Correct your dose by turning counterclockwise.
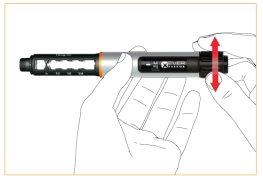
Injection
- With the help of a surgical wipe, clean the area of the skin where you plan to inject the medication and surrounding areas.
- Inject Dacepton into the central area of your waist (abdomen) or the outer part of your thigh, under the skin (subcutaneously) as indicated by your doctor or nurse.
To inject, press the button all the way to the end. Keep the button pressed during the medication discharge. Once the medication has been completely discharged, wait 6 seconds and then slowly remove the pen needle. You can keep the button pressed or release it during the 6 seconds. Check in the window that you see the "0.0" position to confirm that the complete dose has been administered. 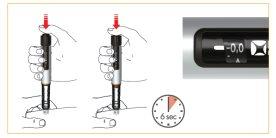
- Change the injection site each time you use Dacepton 10 mg/ml in a cartridge. This will reduce the likelihood of a skin reaction at the injection site. Do not inject Dacepton into an area of skin that is painful, red, infected, or damaged.
- Never inject directly into a vein (intravenously) or muscle (intramuscularly).
After using Dacepton 10 mg/ml in a cartridge
Discard and dispose of the needle after each injection (see section 5 for how to safely dispose of it).
Removing the pen needle after each injection
Carefully place the outer protective cover over the pen needle.
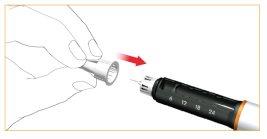
Unscrew the needle from the pen by turning the outer body clockwise and dispose of it correctly.

Optional:
Place the outer cover of the pen needle in the corresponding hole in its case. The opening of the needle cover should point upwards. Carefully insert the needle (attached to the pen) into the opening of the case. Without touching the cover, push down firmly and turn counterclockwise to unscrew the needle from the pen.
Close the pen cap well after each use.
- Leave the cartridge in your pen.
- A new cartridge can be used for a maximum of 15 days (for more information, see section 5 "Storage of Dacepton 10 mg/ml in a cartridge").
- If there is not enough solution for your next dose, discard and dispose of the cartridge.
- Discard the needle in a safe manner, as described in the Instructions for Use of your pen.
If you use more Dacepton 10 mg/ml in a cartridge than you should
- Tell your doctor or contact the emergency service of the nearest hospital immediately.
- You may notice that your heart beats slower, excessive dizziness, excessive sleepiness, and/or difficulty breathing. You may also feel weak or dizzy, especially when standing up, due to low blood pressure. Lying down with your feet elevated can help you combat low blood pressure.
In case of overdose or accidental ingestion, consult your doctor or call the Toxicology Information Service, phone 91 562 04 20, indicating the medication and the amount ingested.
If you forget to use Dacepton 10 mg/ml in a cartridge
Administer the next dose when you need it. Do not use a double dose to make up for forgotten doses.
If you interrupt treatment with Dacepton 10 mg/ml in a cartridge
Do not interrupt treatment with Dacepton without consulting your doctor first.
If you have any other doubts about the use of this medication, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
If you experience an allergic reaction, stopusing Dacepton 10 mg/ml in a cartridge and contact your doctor or the emergency service of the nearest hospital immediately.
The signs of an allergic reaction may include:
- Rash
- Difficulty breathing or
- Chest tightness,
- Swelling of the eyelids, face, or lips,
- Inflammation or redness of the throat or tongue.
Dacepton may sometimes cause the following side effects.
Very common side effects (may affect more than 1 in 10 patients):
- Lumps under the skin at the injection site that are painful, annoying, and may turn red and itch. To avoid these lumps, it is advisable to change the injection site each time you insert the needle.
- Hallucinations (seeing, hearing, or feeling things that do not exist)
Common side effects (may affect up to 1 in 10 patients):
- Nausea or vomiting, especially at the beginning. If you are taking domperidone and still feel nauseous, or if you are not taking domperidone and feel dizzy, tell your doctor or nurse as soon as possible.
- Fatigue or excessive sleepiness.
- Confusion or hallucinations.
- Feeling dizzy or slightly dizzy when standing up
Uncommon side effects (may affect up to 1 in 100 patients):
- Increased involuntary movements or increased tremors during 'on' periods.
- Hemolytic anemia, an abnormal destruction of red blood cells in blood vessels or other parts of the body. This is an uncommon side effect that may occur in patients who also take levodopa.
- Falling asleep suddenly.
- Rash.
- Difficulty breathing.
- Ulcers at the injection site.
- Decrease in red blood cells, which can cause the skin to turn yellow and lead to weakness or shortness of breath.
- Decrease in blood platelets, which increases the risk of bleeding or bruising.
Rare side effects (may affect up to 1 in 1,000 patients):
- An allergic reaction
- Eosinophilia, an abnormally high number of white blood cells in the blood or body tissues.
Side effects of unknown frequency (cannot be estimated from available data):
- Swelling of the legs, feet, or fingers.
- Fainting
- Aggression, agitation
- Headache
- Inability to resist the impulse, desire, or temptation to perform an action that could be harmful to you or others, which may include:
- Strong impulse to gamble excessively, despite serious personal or family consequences.
- Alteration or increase in sexual interest and behavior, significant to you or others, for example, increased sexual impulse.
- Uncontrolled shopping or spending.
- Binge eating (eating large amounts of food in a short time) or compulsive eating (eating more food than normal and more than necessary to satisfy hunger).
Tell your doctor if you experience any of these behaviors; they will indicate how to control or reduce the symptoms.
Reporting side effects:
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if they are possible side effects not listed in this leaflet. You can also report them directly through the Spanish Medication Surveillance System for Human Use: https://www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Dacepton 10 mg/ml in a cartridge
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date shown on the box and label after "EXP". The expiration date is the last day of the month indicated.
Do not store above 25°C.
Do not refrigerate or freeze.
Keep the cartridges in the outer packaging to protect them from light.
Store in the same conditions once opened and between openings.
When you start using a new cartridge, it can be used for up to 15 days. Do not reuse the cartridge after this period. Use a new cartridge.
Do not use this medication if you notice that the solution has turned green. It should only be used if the solution is transparent, colorless, or slightly yellowish and does not contain particles.
Medications should not be thrown away in drains or trash. Deposit the packaging and medications you no longer need in the SIGRE Point of your usual pharmacy. Ask your pharmacist how to dispose of the packaging and medications you no longer need. This will help protect the environment.
6. Package contents and additional information
Composition of Dacepton 5 mg/ml
The active ingredientis apomorphine hydrochloride hemihydrate. Each milliliter of Dacepton 5 mg/ml contains 5 mg of apomorphine hydrochloride hemihydrate.
Dacepton 5 mg/ml is presented in 20 ml vials containing 100 mg of apomorphine hydrochloride hemihydrate.
The other components (excipients) are:
- Sodium metabisulfite (E223)
- Sodium chloride
- Hydrochloric acid (for pH adjustment)
- Water for injectable preparations
See section 2: Dacepton 5 mg/ml contains sodium metabisulfite (E223) and sodium chloride, regarding sodium metabisulfite and sodium chloride.
Appearance of the product and packaging content
Dacepton 5 mg/ml is a perfusion solution, transparent and colorless to slightly yellowish.
Glass vials with 20 ml of perfusion solution, in boxes of 1, 5 or 30 vials.
Packaging sizes: 5 x 1, 10 x 1, 30 x 1, 2 x 5 and 6 x 5
Only some packaging sizes may be marketed.
Marketing authorization holder
EVER Neuro Pharma GmbH
A-4866 Unterach
Austria
Manufacturer
EVER Pharma Jena GmbH
Brüsseler Strasse 18
07747 Jena
Germany
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
EVER Pharma Therapeutics Spain, S.L.
C/Toledo 170
28005 Madrid
Spain
This medication is authorized in the member states of the European Economic Area and in the United Kingdom and Northern Ireland, with the following names:
AT Dacepton® 5mg/ml Infusionslösung
BE Dacepton® 5 mg /ml solution for infusion
BG Dacepton® 5mg/ml ?????????? ???????
CZ Dacepton® 5mg/ml Infuzní roztok
DE Dacepton® 5mg/ml Infusionslösung
DK Dacepton® 5 mg /ml infusion solution
EE Dacepton® 5 mg /ml
EL Dopaceptin® 5 mg /ml Δι?λυμαγια?γχυση
ES Dacepton® 5mg/ml Solución para perfusión
FI Dacepton® 5 mg /ml infuusioneste, liuos
FR Dopaceptin® 5 mg /ml Solution pour perfusion
HU Dacepton® 5 mg /ml Oldatos infúzió
IE Dacepton® 5 mg /ml solution for infusion
IT Dacepton®
LT Dacepton® 5mg/ml Infuzinis tirpalas
LV Dacepton® 5mg/ml škidums infuzijam
NL Dacepton® 5 mg /ml oplossing voor infusie
NO Dacepton®
PL Dacepton®
PT Dacepton®
SE Dacepton® 5 mg /ml infusionsvätska, lösning
SI Dacepton® 5 mg /ml raztopina za infundiranje
SK Dacepton® 5mg/ml Infúzny roztok
UK (Northern Ireland) Dacepton® 5 mg /ml solution for infusion
Date of the last revision of this prospectus: October 2023
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DACEPTON 10 mg/ml Injectable Solution in CartridgeDosage form: INJECTABLE, 10 mg/mLActive substance: apomorphineManufacturer: Stada Arzneimittel AgPrescription requiredDosage form: INJECTABLE, 10 mg/ml apomorphine hydrochlorideActive substance: apomorphineManufacturer: Stada Arzneimittel AgPrescription requiredDosage form: INJECTABLE PERFUSION, 5 mg/mLActive substance: apomorphineManufacturer: Stada Arzneimittel AgPrescription required
Alternatives to DACEPTON 10 mg/ml Injectable Solution in Cartridge in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to DACEPTON 10 mg/ml Injectable Solution in Cartridge in Poland
Alternative to DACEPTON 10 mg/ml Injectable Solution in Cartridge in Ukraine
Online doctors for DACEPTON 10 mg/ml Injectable Solution in Cartridge
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for DACEPTON 10 mg/ml Injectable Solution in Cartridge – subject to medical assessment and local rules.







