
CYRAMZA 10 mg/mL concentrate for solution for infusion

How to use CYRAMZA 10 mg/mL concentrate for solution for infusion
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
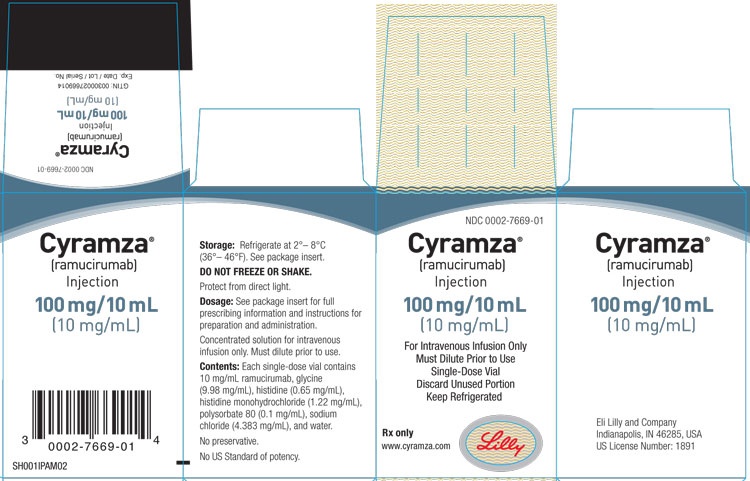
Cyramza 10 mg/ml Concentrate for Solution for Infusion
ramucirumab
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor.
- If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Pack
- What is Cyramza and what is it used for
- What you need to know before you use Cyramza
- How to use Cyramza
- Possible side effects
- Storage of Cyramza
- Contents of the pack and other information
1. What is Cyramza and what is it used for
Cyramza is a cancer medicine that contains ramucirumab as the active substance, which is a monoclonal antibody. It is a specialized protein that can recognize and bind to another protein found in blood vessels called “VEGF Receptor 2”. This receptor is necessary for the development of new blood vessels. For cancer to grow, it needs to develop new blood vessels. By binding to and blocking the “VEGF Receptor 2”, the medicine disrupts the blood supply to cancer cells.
Cyramza is also used in combination with paclitaxel, another anticancer medicine, for the treatment of advanced stomach cancer (or cancer of the junction between the esophagus and the stomach) in adults whose disease has worsened after treatment with cancer medicines.
Cyramza is used for the treatment of advanced stomach cancer (or cancer of the junction between the esophagus and the stomach) in adults whose disease has worsened after treatment with cancer medicines and for whom treatment with Cyramza in combination with paclitaxel is not suitable.
Cyramza is used for the treatment of colon or rectal cancer (parts of the large intestine) in adults. It is administered with another medicine called “FOLFIRI”, which includes “5-fluorouracil”, “folinic acid”, and “irinotecan”.
Cyramza is administered in combination with erlotinib, another anticancer medicine, as first-line therapy for the treatment of adult patients with advanced non-small cell lung cancer when the tumor cells have specific changes (mutations) in the epidermal growth factor receptor gene.
Cyramza is administered in combination with docetaxel, another anticancer medicine, for the treatment of adult patients with advanced lung cancer whose disease has worsened after treatment with cancer medicines.
Cyramza is used to treat liver cancer that is advanced or cannot be removed by surgery, in adults who have been previously treated with another anticancer medicine (sorafenib) and who have a high level of a specific protein in the blood (alpha-fetoprotein).
2. What you need to know before you use Cyramza
Do not use Cyramza:
- if you are allergic to ramucirumab or any of the other ingredients of this medicine (listed in section 6).
- if X-rays show signs that your lung cancer has a cavity or hole or if the lung cancer is attached to major blood vessels.
Warnings and precautions
Consult your doctor or nurse beforestarting to use Cyramza if:
- you have a disease that increases the risk of bleeding. Also, consult your doctor if you are taking other medicines that increase the risk of bleeding or affect the blood's ability to clot. In these cases, your doctor will perform regular blood tests to monitor the risk of bleeding.
- you have liver cancer and have had previous bleeding from varices in your digestive tract (esophagus) or have portal vein hypertension, which carries blood from the intestine and spleen to the liver.
- you have lung cancer and have had recent bleeding in the lung (coughing up bright red blood) or are continuously taking non-steroidal anti-inflammatory medicines or medicines that affect the blood's ability to clot.
- you have high blood pressure. Cyramza may increase the occurrence of high blood pressure. Your doctor will ensure that if you already have high blood pressure, it is under control before starting treatment with Cyramza. During treatment with Cyramza, your doctor will monitor your blood pressure and adjust your antihypertensive medication if necessary. Treatment with Cyramza may be temporarily interrupted until high blood pressure is controlled with medication or permanently interrupted if it cannot be adequately controlled.
- you have or have had an aneurysm (enlargement and weakening of a blood vessel wall) or a tear in the wall of a blood vessel.
- you are going to have surgery, if you have recently had surgery, or if after surgery your wound is not healing well. Cyramza may increase the risk of wound healing problems. You should not receive Cyramza for at least 4 weeks before scheduled surgery, and your doctor will decide when to resume treatment. If you have a wound that is not healing during treatment, the dose of Cyramza will be interrupted until the wound is completely healed.
- you have severe liver disease (“cirrhosis”) and associated conditions such as excessive fluid accumulation in your abdomen (“ascites”). Your doctor will discuss with you whether the potential benefits of treatment justify the increased risk for you. If you have liver cancer, your doctor will monitor you for signs and symptoms of confusion and/or disorientation associated with chronic liver problems and will suspend treatment with Cyramza if you develop these signs and symptoms.
- you have severe kidney problems. The available data on the use of Cyramza in patients with severe renal impairment are limited.
Consult your doctor or nurse immediatelyif any of the following apply to you (or you are not sure) during treatmentwith Cyramza or at any other time after:
- Blockage of the arteries by a blood clot(“arterial thromboembolic disease”):
Cyramza may cause a blood clot in your arteries. Blood clots in the arteries can cause serious diseases, including heart attack or stroke. The symptoms of a heart attack can include chest pain or pressure. The symptoms of a stroke can include sudden numbness or weakness of the arm, legs, or face, confusion, difficulty speaking or understanding others, sudden difficulty walking or loss of balance or coordination or sudden dizziness. If you develop a blood clot in your arteries, treatment with Cyramza will be permanently interrupted.
- Perforation of the intestine wall(“gastrointestinal perforation”): Cyramza may increase the risk of developing perforations in the wall of your intestine. The symptoms include severe abdominal pain, vomiting, fever, or chills. If you develop a perforation in the intestine wall, treatment with Cyramza will be permanently interrupted.
- Severe bleeding:Cyramza may increase the risk of severe bleeding. The symptoms can include extreme tiredness, weakness, dizziness, or changes in the color of your stool. If you experience severe bleeding, treatment with Cyramza will be permanently interrupted.
- Infusion-related reactions:Infusion-related reactions can occur during treatment due to the fact that Cyramza is administered by intravenous infusion (see section 3). Your doctor or nurse will monitor you for adverse effects during the infusion. The symptoms can include increased muscle tension, back pain, pain and/or pressure in the chest, chills, flushing, difficulty breathing, wheezing, and a feeling of tingling or numbness in the hands or feet. In severe cases, the symptoms can include difficulty breathing caused by narrowing of the airways, rapid heartbeat, and a feeling of fainting. If you experience a severe infusion-related reaction, treatment with Cyramza will be permanently interrupted.
- A rare but serious brain diseasecalled “posterior reversible encephalopathy syndrome” or “PRES” (by its English acronym): Cyramza may increase the risk of developing this brain disease. The symptoms can include seizures, headache, feeling sick (nausea), vomiting, blindness, or decreased level of consciousness, with or without high blood pressure. Interrupt treatment with Cyramza if you experience this brain disease.
- Heart failure:Cyramza, when administered in combination with chemotherapy or erlotinib, may increase the risk of heart failure. The symptoms can include weakness and tiredness, swelling, and fluid accumulation in the lungs, which can cause difficulty breathing. Your symptoms will be assessed, and it may be considered to suspend your treatment with Cyramza.
- Abnormal connections within the body(“fistula”): Cyramza may increase the risk of developing abnormal connections within the body between internal organs and the skin or other tissues. If you develop a fistula, treatment with Cyramza will be permanently interrupted.
- Abnormal urine test(“proteinuria”): Cyramza may increase the risk of developing or worsening abnormal protein levels in the urine. Treatment with Cyramza may need to be temporarily interrupted until protein levels in the urine decrease and then resumed at a lower dose, or permanently interrupted if protein levels in the urine do not decrease sufficiently.
- Mouth inflammation(“stomatitis”): Cyramza, when administered in combination with chemotherapy, may increase the risk of developing mouth inflammation. The symptoms can include a burning sensation in the mouth, ulcers, blisters, or inflammation. Your doctor may prescribe a treatment to help you with the symptoms.
- Fever or infection:During treatment, you may have a temperature of 38°C or higher (since you may have a lower than normal number of white blood cells, which is very common). The symptoms can include sweating or other signs of infection, such as headache, pain in the limbs, or decreased appetite. The infection (sepsis) could be severe and lead to death.
- Elderly patients with lung cancer:Your doctor will carefully consider the most appropriate treatment for you.
Children and adolescents
Cyramza must not be administered to patients under 18 years of age because there is no information on how it works in this age group.
Using Cyramza with other medicines
Tell your doctor if you are using, have recently used, or might use any other medicines. This includes medicines obtained without a prescription and herbal medicines.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine. You must avoid becoming pregnant while receiving this medicine and for at least 3 months after receiving the last dose of Cyramza. Consult your doctor about the best contraceptive method for you.
As Cyramza prevents the development of new blood vessels, it may reduce the likelihood of becoming pregnant or maintaining a pregnancy. It may also harm the fetus. You must not use this medicine during pregnancy. If you become pregnant during treatment with Cyramza, your doctor will discuss with you whether the benefits of treatment are greater than any possible risk to you or your baby.
It is not known whether the medicine passes into breast milk and could affect the baby. Therefore, you must not breastfeed your baby during treatment with Cyramza and for at least 3 months after receiving the last dose.
Driving and using machines
The influence of Cyramza on the ability to drive and use machines is negligible. If you experience any symptoms that affect your ability to concentrate and react, do not drive or use machines until the symptoms disappear.
Cyramza contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) in each 10 ml vial; this is essentially “sodium-free”.
This medicine contains approximately 85 mg of sodium (the main component of table/cooking salt) in each 50 ml vial. This is equivalent to approximately 4% of the maximum recommended daily intake of sodium for an adult.
3. How to use Cyramza
This cancer treatment will be administered to you by your doctor or nurse.
Dose and frequency of administration
The appropriate amount of Cyramza needed to treat your disease will be calculated by your doctor or hospital pharmacist based on your body weight.
The recommended dose of Cyramza for the treatment of stomach cancer, for the treatment of advanced colon or rectal cancer, and for the treatment of liver cancer is 8 mg per kilogram of body weight every 2 weeks.
The recommended dose of Cyramza for the treatment of lung cancer is 10 mg per kilogram of body weight every 2 weeks when administered in combination with erlotinib or every 3 weeks when administered in combination with docetaxel.
The number of infusions you receive will depend on how you respond to treatment. Your doctor will discuss this with you.
Pre-medication
You may receive another medicine to reduce the risk of infusion-related reactions before receiving Cyramza. If you experience an infusion-related reaction during treatment with Cyramza, you must receive pre-medication for all subsequent infusions.
Dose adjustment
During each infusion, your doctor or nurse will check if you experience any adverse effects.
If you experience an infusion-related reaction during treatment, the final duration of the infusion will be increased for the remainder of that infusion and for subsequent infusions.
The amount of protein in the urine will be regularly checked during treatment. Cyramza may be temporarily interrupted based on the measurement of protein levels. Once protein levels in the urine have decreased to certain levels, treatment can be resumed at a lower dose.
Method and route of administration
Cyramza is a concentrate for solution for infusion (also called “sterile concentrate”). A hospital pharmacist, nurse, or doctor will dilute the contents of the vial in a sodium chloride 9 mg/ml (0.9%) solution before use. This medicine is administered by infusion over approximately 60 minutes.
Treatment with Cyramza will be temporarily interrupted if:
- you experience high blood pressure, until it is controlled with antihypertensives
- you experience wound healing problems, until the wound is healed
- you have scheduled surgery, 4 weeks before surgery
- you experience a blood clot in your arteries
- you experience a perforation in the intestine wall
- you experience severe bleeding
- you experience a severe infusion-related reaction
- you experience high blood pressure that cannot be controlled with medication
- you are passing more protein than normal in your urine or experience severe kidney disease (nephrotic syndrome)
- you develop an abnormal connection within your body between internal organs and the skin or other tissues (fistula)
- you develop confusion and/or disorientation associated with chronic liver problems
- decreased kidney function (in the context of liver failure)
- Perforations in the intestinal wall:Perforation that develops in the stomach or intestine. Symptoms include severe abdominal pain, vomiting, fever, or chills.
- Severe bleeding in the intestine:symptoms may include extreme fatigue, weakness, dizziness, or changes in the color of your stools.
- Thrombi in arteries:thrombi in the arteries can cause a heart attack or cerebral embolism. Symptoms of a heart attack may include pain or pressure in the chest. Symptoms of a cerebral embolism may include sudden numbness or weakness of the arm, legs, or face, confusion, difficulty speaking or understanding others, sudden difficulty walking, or loss of balance or coordination, or sudden dizziness.
- A brain diseasecalled posterior reversible encephalopathy syndrome: symptoms may include seizures (convulsions), headache, feeling sick (nausea), vomiting, blindness, or decreased level of consciousness, with or without high blood pressure.
- fatigue or weakness
- low white blood cell count in the blood (may increase the risk of infection)
- infections
- diarrhea
- hair loss
- nasal bleeding
- inflammation of the inside of the mouth
- high blood pressure
- reduction in the number of red blood cells, which can cause pale skin
- swelling of hands, feet, and legs due to fluid retention
- low platelet count (blood cells that help blood clot)
- abdominal pain
- protein in the urine (abnormal urine test)
- headache
- inflammation of the mucous membranes, such as the digestive and respiratory tracts
- fever accompanied by low white blood cell count
- low levels of a protein called albumin in the blood
- reactions related to the infusion site
- rash
- erythema, inflammation, numbness/tingling, or pain and/or peeling of the skin of the hands and/or feet (called hand-foot syndrome)
- hoarseness
- bleeding in the lungs
- low sodium levels in the blood (hyponatremia) that can cause fatigue and confusion or muscle spasms
- bleeding from the gums
- confusion and/or disorientation in patients with chronic liver problems
- intestinal obstruction; symptoms may include constipation and abdominal pain
- underactive thyroid gland that can cause fatigue or weight gain (hypothyroidism)
- abnormal growth of blood vessels
- severe infections (sepsis)
- low potassium levels in the blood (hypokalemia) that can cause muscle weakness, spasms, or abnormal heart rhythm
- heart condition in which the heart muscle does not pump blood as well as it should, causing difficulty breathing and swelling of the legs and feet
- abnormal blood clotting in small blood vessels
- weakening and rupture of a blood vessel wall or tear of a blood vessel wall (aneurysms and arterial dissections)
- The active substance is ramucirumab. One milliliter of concentrate for solution for infusion contains 10 mg of ramucirumab.
- Each 10 ml vial contains 100 mg of ramucirumab.
- Each 50 ml vial contains 500 mg of ramucirumab.
- The other ingredients are histidine, histidine monohydrochloride, sodium chloride, glycine (E640), polysorbate 80 (E433), and water for injectable preparations (see section 2 "Cyramza contains sodium").
- 1 vial of 10 ml
- 2 vials of 10 ml
- 1 vial of 50 ml
Treatment with Cyramza will be permanently interrupted if:
When you receive Cyramza in combination with paclitaxel or docetaxel
Paclitaxel and docetaxel are administered by intravenous infusion over a period of approximately 60 minutes. If you receive Cyramza in combination with paclitaxel or docetaxel on the same day, Cyramza will be administered first.
The amount of paclitaxel or docetaxel needed depends on your body surface area. Your doctor or hospital pharmacist will calculate your body surface area by measuring your height and weight and calculate the appropriate dose for you.
The recommended dose of paclitaxel is 80 mg per square meter (m2) of your body surface area once a week for 3 weeks followed by 1 week without treatment.
The recommended dose of docetaxel is 75 mg per square meter (m2) of your body surface area once every 3 weeks. If you are of East Asian origin, you may receive a reduced initial dose of docetaxel of 60 mg per m2 of your body surface area once every 3 weeks.
Before starting any infusion with paclitaxel, a blood test will be performed to check that your blood cell count is sufficiently high and that your liver is functioning properly.
Read the package leaflet of paclitaxel or docetaxel for more information.
When you receive Cyramza in combination with FOLFIRI
Chemotherapy with FOLFIRI is administered by intravenous infusion once the Cyramza infusion is complete. Please read the package leaflet of the other medicines that are part of your treatment to check if they are suitable for you. If you are not sure, ask your doctor, pharmacist, or nurse if there is any reason why you cannot use these medicines.
When you receive Cyramza in combination with erlotinib
Please read the package leaflet of erlotinib for more information about erlotinib and to check if it is suitable for you. If you are not sure, ask your doctor, pharmacist, or nurse if there is any reason why you cannot take erlotinib.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Consult your doctor immediatelyif you suffer from any of the following serious adverse effects that have been observed during treatment with Cyramza (see also What you need to know before startingto use Cyramza):
Frequent Adverse Effects(may affect up to 1 in 10 people):
Rare Adverse Effects(may affect up to 1 in 1,000 people):
Consult your doctor if you experience any of the following adverse effects:
Very Frequent Adverse Effects(may affect more than 1 in 10 people):
Frequent Adverse Effects(may affect up to 1 in 10 people):
Infrequent Adverse Effects(may affect up to 1 in 100 people):
Rare Adverse Effects(may affect up to 1 in 1,000 people):
Unknown Frequency(frequency cannot be estimated from available data):
Cyramza may cause changes in laboratory tests. These changes may be among the adverse effects mentioned above: low white blood cell count in the blood, low platelet count in the blood, low levels of albumin, potassium, or sodium in the blood, presence of protein in the urine.
Reporting Adverse Effects
If you experience any adverse effect, consult your doctor, even if it is a possible adverse effect not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can help provide more information on the safety of this medicine.
5. Storage of Cyramza
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the carton and vial label after EXP. The expiration date is the last day of the month indicated.
Store in a refrigerator (between 2°C and 8°C).
Do not freeze.
Keep the vial in the outer packaging to protect it from light.
Do not freeze or shake the infusion solution. Do not administer the solution if you notice any particles or unusual color.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Package Contents and Additional Information
Cyramza Composition
Appearance of the Product and Package Contents
The concentrate for solution for infusion (or sterile concentrate) is a clear to slightly opalescent solution with a colorless to slightly yellowish tint, presented in a glass vial with a rubber stopper.
Cyramza is available in packs of:
Not all pack sizes may be marketed.
Marketing Authorization Holder
Eli Lilly Nederland B.V.
Papendorpseweg 83
3528 BJ Utrecht
Netherlands
Manufacturer
Lilly, S.A.
Avda de la Industria, 30
Alcobendas
28108 Madrid
Spain
Lilly France Fegersheim
2 rue du Colonel Lilly
67640 Fegersheim
France
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Belgium/België/BelgienLithuania
Eli Lilly Benelux S.A./N.V. Eli Lilly Lietuva
Tel: + 32-(0)2 548 84 84 Tel: +370 (5) 2649600
BulgariaLuxembourg/Luxemburg
Eli Lilly Bulgaria EOOD Eli Lilly Benelux S.A./N.V.
Tel: + 359 2 491 41 40 Tel: + 32-(0)2 548 84 84
Czech RepublicHungary
ELI LILLY CR, s.r.o. Lilly Hungária Kft.
Tel: + 420 234 664 111 Tel: + 36 1 328 5100
DenmarkMalta
Eli Lilly Danmark A/S Charles de Giorgio Ltd.
Tel: +45 45 26 60 00 Tel: + 356 25600 500
GermanyNetherlands
Lilly Deutschland GmbH Eli Lilly Nederland B.V.
Tel: + 49-(0) 6172 273 2222 Tel: + 31-(0) 30 60 25 800
EstoniaNorway
Eli Lilly Nederland B.V. Eli Lilly Norge A.S.
Tel: +372 6 817 280 Tel: + 47 22 88 18 00
GreeceAustria
ΦΑΡΜΑΣΕΡΒ-ΛΙΛΛΥ Α.Ε.Β.Ε. Eli Lilly Ges.m.b.H.
Tel: +30 210 629 4600 Tel: + 43-(0) 1 711 780
SpainPoland
Lilly S.A. Eli Lilly Polska Sp. z o.o.
Tel: + 34-91 663 50 00 Tel: +48 22 440 33 00
FrancePortugal
Lilly France SAS Lilly Portugal Produtos Farmacêuticos, Lda
Tel: +33-(0) 1 55 49 34 34 Tel: + 351-21-4126600
CroatiaRomania
Eli Lilly Hrvatska d.o.o. Eli Lilly România S.R.L.
Tel: +385 1 2350 999 Tel: + 40 21 4023000
IrelandSlovenia
Eli Lilly and Company (Ireland) Limited Eli Lilly farmacevtska družba, d.o.o.
Tel: + 353-(0) 1 661 4377 Tel: +386 (0)1 580 00 10
IcelandSlovak Republic
Icepharma hf. Eli Lilly Slovakia s.r.o.
Tel: + 354 540 8000 Tel: + 421 220 663 111
ItalyFinland
Eli Lilly Italia S.p.A. Oy Eli Lilly Finland Ab
Tel: + 39- 055 42571 Tel: + 358-(0) 9 85 45 250
CyprusSweden
Phadisco Ltd Eli Lilly Sweden AB
Tel: +357 22 715000 Tel: + 46-(0) 8 7378800
LatviaUnited Kingdom
Eli Lilly (Suisse) S.A Representative Office Latvia Eli Lilly and Company Limited
Tel: +371 67364000 Tel: + 44-(0) 1256 315000
Date of the Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
---------------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Do not shake the vial.
Prepare the infusion solution using aseptic techniques to ensure the sterility of the prepared solution.
Each vial is for single use. Before dilution, the contents of the vials should be checked for the possible presence of particles or discoloration (the concentrate for solution for infusion should be clear to slightly opalescent and colorless to slightly yellowish without visible particles). If particles or color changes are identified, the vial should be discarded.
Calculate the dose and volume of ramucirumab needed to prepare the infusion solution. The vials contain 100 mg or 500 mg in a solution of 10 mg/ml of ramucirumab. Only use sodium chloride 9 mg/ml (0.9%) injectable solution as a diluent.
In case of using a pre-filled infusion bag
According to the calculated volume of ramucirumab, remove the corresponding volume of sodium chloride 9 mg/ml (0.9%) injectable solution from the pre-filled 250 ml infusion bag.
The transfer of the calculated volume of ramucirumab to the infusion bag should be performed aseptically. The final total volume of the bag should be 250 ml. The bag should be carefully inverted to ensure adequate mixing. DO NOT FREEZE OR SHAKE the infusion solution. DO NOT dilute with other solutions or co-administer with other medicines or electrolytes.
In case of using an empty infusion bag
The transfer of the calculated volume of ramucirumab to the empty infusion bag should be performed aseptically. Add a sufficient amount of sodium chloride 9 mg/ml (0.9%) injectable solution to the bag to achieve a total volume of 250 ml. The bag should be carefully inverted to ensure adequate mixing. DO NOT FREEZE OR SHAKE. DO NOT dilute with other solutions or co-administer with other medicines or electrolytes.
After dilution and preparation, the medicine should be used immediately. If not used immediately, the time and conditions of storage prior to use are the responsibility of the user and should not normally exceed 24 hours between 2°C and 8°C.
Parenteral medicines should be visually inspected before administration to rule out the presence of particles. If particles are identified, the vial should be discarded.
Discard any remaining ramucirumab in the vial since the medicine does not contain antimicrobial preservatives.
Administer through an infusion pump. A separate infusion line with a 0.22-micron low-protein-binding in-line filter should be used for infusion, and the line should be flushed with sodium chloride 9 mg/ml (0.9%) injectable solution after completion of the infusion.
The disposal of unused medicine and all materials that have come into contact with it will be carried out in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CYRAMZA 10 mg/mL concentrate for solution for infusionDosage form: INJECTABLE PERFUSION, 10 mg/mlActive substance: ramucirumabManufacturer: Eli Lilly Nederland B.V.Prescription requiredDosage form: INJECTABLE PERFUSION, 25 mg/mlActive substance: bevacizumabManufacturer: Biosimilar Collaborations Ireland LimitedPrescription requiredDosage form: INJECTABLE PERFUSION, 25 mg/mlActive substance: bevacizumabManufacturer: Mabxience Research S.L.Prescription required
Online doctors for CYRAMZA 10 mg/mL concentrate for solution for infusion
Discuss questions about CYRAMZA 10 mg/mL concentrate for solution for infusion, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






