
CUROSURF 240

How to use CUROSURF 240
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What Curosurf 240 mg Suspension for Endotracheal Administration is and what it is used for
- What you need to know before you use Curosurf 240 mg Suspension for Endotracheal Administration
- How to use Curosurf 240 mg Suspension for Endotracheal Administration
- Possible side effects
- Storage of Curosurf 240 mg Suspension for Endotracheal Administration
- Contents of the pack and other information
Introduction
Package Leaflet: Information for the User
CUROSURF 240 mg
Suspension for Endotracheal Administration
Porcine Lung Surfactant
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
Contents of the Package Leaflet:
- What Curosurf 240 mg Suspension for Endotracheal Administration is and what it is used for
- What you need to know before you use Curosurf 240 mg Suspension for Endotracheal Administration
- How to use Curosurf 240 mg Suspension for Endotracheal Administration
- Possible side effects
- Storage of Curosurf 240 mg Suspension for Endotracheal Administration
- Contents of the pack and other information
1. What Curosurf 240 mg Suspension for Endotracheal Administration is and what it is used for
Curosurf is used to treat or prevent Respiratory Distress Syndrome (RDS) in newborn babies. Most newborn babies are born with a substance in their lungs called "surfactant". This substance coats the lungs and prevents them from sticking together, making it easier to breathe. However, some babies, especially premature babies, do not have enough of this surfactant when they are born, which causes RDS. Curosurf is a natural surfactant that works in the same way as the baby's own surfactant, helping the baby to breathe normally until they can produce surfactant on their own.
2. What you need to know before you use Curosurf 240 mg Suspension for Endotracheal Administration
Do not use Curosurf 240 mg Suspension for Endotracheal Administration
- If you are allergic to porcine lung surfactant or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
The baby's general condition should be stabilized. It is also recommended to correct acidosis, hypotension, anemia, hypoglycemia, and hypothermia.
Babies born after a very prolonged period after rupture of membranes (more than 3 weeks) may not respond optimally.
Surfactant administration may decrease the severity of RDS but does not completely eliminate mortality and morbidity associated with prematurity, as these babies have other complications. After Curosurf administration, a depression of cerebral electrical activity has been detected, lasting 2-10 minutes, whose impact is not well known.
Curosurf has not been studied in preterm babies with severe hypotension.
Children
This medicine is only for premature babies.
Interaction of Curosurf 240 mg Suspension for Endotracheal Administration with other medicines
Tell your doctor or pharmacist if you are using or have recently used other medicines, including those obtained without a prescription, homeopathic medicines, herbal remedies, and other health-related products, as it may be necessary to interrupt treatment or adjust the dose of one of them.
However, no interactions have been observed between Curosurf and the medicines commonly used in Intensive Care Units for premature babies.
Pregnancy and breastfeeding
This medicine is only for premature babies.
Driving and using machines
This is not applicable, as this medicine is only for premature babies.
Curosurf 240 mg Suspension for Endotracheal Administration contains sodium
This medicine contains less than 23 mg (1 mmol) of sodium per vial, so it is considered essentially "sodium-free".
3. How to use Curosurf 240 mg Suspension for Endotracheal Administration
Follow the instructions for administration of this medicine exactly as indicated by your doctor. If you are in doubt, consult your doctor again.
Curosurf should only be administered by trained personnel with experience in the care, resuscitation, and stabilization of premature babies, and only when there are adequate facilities for ventilation and monitoring of babies with Respiratory Distress Syndrome.
Curosurf 240 mg Suspension for Endotracheal Administration is administered via the endotracheal route.
The recommended dose for treatment is:
- 200 mg/kg (1.25-2.5 ml/kg), administered as a single dose or divided into 2 doses of 100 mg/kg, the first of which should be administered immediately after diagnosis of RDS and the second after 12 hours.
- If necessary, an additional dose of 100 mg/kg can be administered after an interval of 12 hours in newborns who continue to require assisted ventilation and supplemental oxygen. The maximum total dose is 300-400 mg/kg.
The recommended dose for prevention (prophylaxis) is:
- 100-200 mg/kg (1.25-2.5 ml/kg), administered as soon as possible after birth (preferably within the first 15 minutes) as a single dose.
- If necessary, an additional dose of 100 mg/kg can be administered 6-12 hours after the first dose and repeated 12 hours later if signs of RDS persist and assisted ventilation is still required. The maximum total dose is 300-400 mg/kg.
If you use more Curosurf 240 mg Suspension for Endotracheal Administration than you should
No cases of overdose have been reported after administration of Curosurf. However, in the unlikely event of an accidental overdose, and only if there are clear clinical effects on respiration, ventilation, or oxygenation of the newborn, as much of the suspension as possible should be aspirated and supportive treatment should be administered, with special attention to hydroelectrolytic balance.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount ingested.
If you have any further questions about the use of this product, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Curosurf 240 mg Suspension for Endotracheal Administration can cause side effects, although not everybody gets them.
These side effects include:
- Infections and infestations: sepsis (severe infection)
- Nervous system disorders: intracranial hemorrhage
- Respiratory disorders: pneumothorax, pulmonary hemorrhage, chronic lung disease (bronchopulmonary dysplasia), apnea, oxygen toxicity (hyperoxia), cyanosis.
- Cardiac disorders: bradycardia.
- Vascular disorders: hypotension.
- Investigations: reduced oxygen saturation, abnormal electroencephalogram.
- Trauma, poisoning, and procedural complications: endotracheal intubation complication.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly to the Spanish Medicines and Healthcare Products Agency (AEMPS) via their website: https://www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Curosurf 240 mg Suspension for Endotracheal Administration
Store in a refrigerator (2-8°C), protected from light.
Unopened and unused vials of Curosurf that have been at room temperature for up to 24 hours can be returned to the refrigerator for later use. Do not move from room temperature to refrigerator more than once.
Keep out of the reach and sight of children.
Do not use Curosurf after the expiration date stated on the packaging after EXP. The expiration date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Return the packaging and any unused medicine to a pharmacy for proper disposal. If you are unsure, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and other information
Composition of Curosurf 240 mg Suspension for Endotracheal Administration
- The active substance is porcine lung surfactant. Each single-dose vial contains 240 mg of porcine lung surfactant.
- The other ingredients are sodium chloride, sodium bicarbonate, and water for injection.
Appearance of the product and pack contents
A pack containing a 5 ml glass vial with 3 ml of sterile surfactant suspension (80 mg/ml) of white to yellowish color; the stopper is made of chlorobutyl rubber and the cap is made of plastic and aluminum.
Marketing authorization holder and manufacturer
Marketing authorization holder:
CHIESI ESPAÑA, S.A.U.
Plaça d’Europa, 41-43, Planta 10
08908 L’Hospitalet de Llobregat
Barcelona (Spain)
Manufacturer:
CHIESI FARMACEUTICI, S.p.A.
Via San Leonardo 96 - Via Palermo, 26/A
43122 Parma (Italy)
This leaflet was approved in October 2016.
Detailed and up-to-date information on this medicine is available on the website of the Spanish Medicines and Healthcare Products Agency (AEMPS): http://www.aemps.gob.es/.
-----------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals
Method of use
BEFORE using CUROSURF
Aspirate the endotracheal tube before instillation to reduce the risk of obstruction, as changes in ventilation parameters during or immediately after instillation may indicate obstruction of the endotracheal tube by mucus, especially if pulmonary secretions were abundant before administration of the medicine. If obstruction of the endotracheal tube by mucus is suspected and cannot be cleared by aspiration, the endotracheal tube should be replaced immediately.
Method of administration
Warm the vial to room temperature by simply holding it in your hand for a few minutes.
Gently invert the vial several times for a few minutes, WITHOUT SHAKING, until the suspension appears homogeneous.
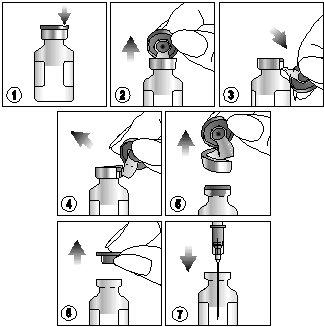
To extract the suspension, carefully follow the instructions below:
- Locate the notch (FLIP UP) on the colored plastic cap.
- Lift the notch and pull upwards.
- Pull the plastic cap with the aluminum portion downwards.
- And 5) Remove the entire ring by tearing off the aluminum wrapper.
- Remove the rubber stopper.
- Extract the contents using a sterile needle and syringe and administer CUROSURF according to the most appropriate modality described below.
CUROSURF can be administered according to the following different modalities:
- Disconnecting the baby from assisted ventilation:
Temporarily disconnect the baby from assisted ventilation and administer 1.25 to 2.5 ml/kg (100-200 mg/kg) of the suspension as a single bolus directly into the lower part of the trachea through the endotracheal tube. Maintain assisted ventilation with an ambu bag for approximately one minute and then reconnect the baby to assisted ventilation under the same conditions as before administration. If additional doses are needed (1.25 ml/kg = 100 mg/kg), they can be administered in the same way.
- Without disconnecting the baby from assisted ventilation:
Administer 1.25 to 2.5 ml/kg (100-200 mg/kg) of the suspension as a single bolus directly into the lower part of the trachea through a catheter passed through the suction port and into the endotracheal tube. If additional doses are needed (1.25 ml/kg = 100 mg/kg), they can be administered in the same way.
- There is a third option for administration through intubation of the newborn to administer the surfactant. The doses are the same as those indicated in sections a and b. In this case, an intubation-extubation technique is used, and after administration of the surfactant and extubation, nasal CPAP (Continuous Positive Airway Pressure) can be applied.
- Less Invasive Surfactant Administration (LISA) using a thin catheter
In premature babies who are breathing spontaneously, Curosurf can also be administered through the Less Invasive Surfactant Administration (LISA) technique using a thin catheter. The doses are the same as those indicated in the administration modalities mentioned in sections a, b, and c. A small-diameter catheter is placed in the trachea of the babies on CPAP, ensuring continuous spontaneous breathing, with direct visualization of the vocal cords by laryngoscopy. Curosurf is instilled in a single bolus over 0.5-3 minutes. After instillation of Curosurf, the tube is immediately removed. CPAP treatment should be maintained throughout the process.
For surfactant administration, a thin catheter with a CE mark for surfactant administration should be used.
DURING treatment, regardless of the administration modality used, the following is recommended:
- Frequent monitoring of blood gases after administration, as an immediate increase in PaO2 or oxygen saturation is normally observed. To maintain adequate oxygen values in the blood, periodic blood gas analysis, continuous monitoring of transcutaneous PO2, or oxygen saturation is recommended.
- Monitoring to identify signs of infection. At the first symptoms of neonatal infection, appropriate antibiotic therapy should be administered.
- Avoiding sudden changes in PaO2 after instillation by immediately adjusting the ventilator. The occurrence of intracranial hemorrhages after Curosurf administration has been associated with a reduction in mean arterial blood pressure and early peaks in arterial oxygenation (PaO2).
AFTER administration of the medicine:
- Do not aspirate tracheal secretions for at least 6 hours, unless there is a vital risk;
- it may be necessary:
- a reduction in peak inspiratory pressure (without waiting for confirmation of blood gas control, if lung expansion improves rapidly);
- a rapid adjustment of the inspired oxygen concentration (to avoid hyperoxia, if there is a rapid increase in arterial oxygen concentration)
INTERRUPT therapy with CUROSURF in case of:
- bradycardia, hypotension, and low oxygen saturation: in these situations, measures should be taken to normalize heart rate. After stabilization, monitoring of the newborn's vital signs should continue.
- reflux: if necessary, the peak inspiratory pressure of the ventilator should be increased to eliminate the obstruction of the endotracheal tube
To complete the picture of complications of prematurity, the following disorders directly related to the severity of the disease and the use of mechanical ventilation, necessary for reoxygenation, may occur: pneumothorax, interstitial pulmonary emphysema, and pulmonary hemorrhage.
Finally, prolonged use of high oxygen concentrations and mechanical ventilation is associated with the development of bronchopulmonary dysplasia and retinopathy of prematurity.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CUROSURF 240Dosage form: ENDOTRACHEOPULMONARY INHALATION, 120 mgActive substance: natural phospholipidsManufacturer: Chiesi España S.A.U.Prescription requiredDosage form: PULMONARY INHALATION, UnknownActive substance: nitric oxideManufacturer: Linde Healthcare AbPrescription requiredDosage form: PULMONARY INHALATION, UnknownActive substance: nitric oxideManufacturer: Linde Healthcare AbPrescription required
Online doctors for CUROSURF 240
Discuss questions about CUROSURF 240, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















