
COLIRCUSI GENTADEXA 5 mg/mL + 1 mg/mL + 0.5 mg/mL EYE/EAR DROPS SOLUTION


How to use COLIRCUSI GENTADEXA 5 mg/mL + 1 mg/mL + 0.5 mg/mL EYE/EAR DROPS SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: information for the user
COLIRCUSÍ GENTADEXA 5 mg/ml + 1 mg/ml + 0.5 mg/ml eye/ear drops in solution
gentamicin sulfate/dexamethasone sodium phosphate/tetrizoline hydrochloride
Read the entire leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the leaflet
- What COLIRCUSÍ GENTADEXA is and what it is used for
- What you need to know before starting to use COLIRCUSÍ GENTADEXA
- How to use COLIRCUSÍ GENTADEXA
- Possible side effects
- Storage of COLIRCUSÍ GENTADEXA
- Package contents and additional information
1. What COLIRCUSÍ GENTADEXA is and what it is used for
It is an association of treatments for inflammation and infection in the eye and ear. It contains as active ingredients gentamicin, an antibiotic belonging to a group called aminoglycosides used to treat infections, dexamethasone, a corticosteroid with anti-inflammatory and antiallergic properties, and tetrizoline, with decongestant action (produces narrowing of the visible blood vessels of the eye); three components that work together to treat infection and inflammation.
One of the active substances of this medication is an antibiotic. Antibiotics are used to treat bacterial infections and are not effective against viral infections.
It is essential that you follow the instructions regarding dosage, administration interval, and treatment duration indicated by your doctor.
Do not store or reuse this medication. If you have any leftover antibiotic after finishing treatment, return it to the pharmacy for proper disposal. Do not throw away medications down the drain or in the trash.
Colircusí Gentadexa is indicated for the treatment of infections of the anterior pole of the eye with inflammatory complications that respond to corticosteroids, caused by germs sensitive to gentamicin. Such as conjunctivitis, i.e., infection of the conjunctiva (transparent membrane that covers the eye) and blepharoconjunctivitis (inflammation of the eyelid) infectious and allergic. Also for keratitis, i.e., inflammation of the cornea or transparent layer that covers the front part of the eye (of superficial, deep, certain hypersensitivity reaction, or phlyctenular type, sclerosing, rosacea acne) and for the treatment of inflammation of the white of the eye, deep (scleritis) and superficial (episcleritis).
Colircusí Gentadexa is also used for the treatment of ear infections (otic) such as allergic external otitis and in all conditions where corticosteroid-antibiotic treatment is required.
2. What you need to know before starting to use COLIRCUSÍ GENTADEXA
Do not use COLIRCUSÍ GENTADEXA
If you are allergic to gentamicin, dexamethasone, tetrizoline, or any other component of this medication (including those listed in section 6).
Do not use Colircusí Gentadexa in your eyes:
- If you have or think you may have:
- Simple glaucoma.
- Narrow-angle glaucoma or narrow angle in your eyes due to anatomy (with blockage of drainage channels).
- Smallpox, chickenpox, or any other eye infection caused by a virus.
- Fungal (fungal) diseases of the eye or untreated eye infections caused by parasites.
- Eye tuberculosis.
- After removing a foreign body from the cornea.
Do not use Colircusí Gentadexa in your ears:
- If you have or think you may have:
- Infection in the ear canal caused by fungi or viruses, or untreated infections caused by parasites.
- Damaged or even perforated eardrum.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Colircusí Gentadexa.
- Use this medication only in your eye(s) or ear(s).
- Due to the risk of possible systemic effects, be especially careful in children, if you are elderly or have a history of severe heart disease, such as hypertension, arteriosclerosis, coronary disease, aneurysm (bulge in the walls of an artery or vein), or other cardiovascular diseases or if you are prone to them, such as if you are diabetic; if you often suffer from low blood pressure when changing posture or have hyperthyroidism, or a tumor of the adrenal gland (pheochromocytoma).
- Due to the presence of tetrizoline, you may experience pupil dilation, increased eye pressure, and systemic adverse effects due to absorption. Cases of effects such as headache, increased blood pressure, alterations in heart rhythm (extrasystoles), rapid heartbeat, dizziness, and stroke have been reported. Precaution is required in case you are being administered certain anesthetics (e.g., halothane).
- If you are using monoamine oxidase inhibitors (MAOIs) or in the last 15 days, you may experience a pronounced increase in blood pressure with this medication. Also with other products that increase tension.
- Severe adverse reactions such as neurotoxicity, ototoxicity, and nephrotoxicity have been observed with aminoglycoside antibiotics used systemically (internally, e.g., orally) or when they are applied locally to open wounds or damaged skin.
- If you experience allergic reactions such as eyelid itching, swelling, or redness, discontinue treatment and consult your doctor. Allergic reactions can range from localized itching or redness to severe allergic reactions (anaphylactic reaction) or severe skin reactions. These allergic reactions can occur with other topical or systemic antibiotics of the same family (aminoglycosides).
- If you are using another antibiotic treatment, consult your doctor.
- If your symptoms worsen or reappear, consult your doctor. With long-term use of this medication, you may become more sensitive to eye or ear infections, such as fungal infections; in case of this type of infection, you should discontinue treatment.
If you develop another infection, your doctor will indicate the treatment to treat it.
- If you use this medication for a long time in your eyes, you may:
- Suffer from increased eye pressure and/or glaucoma. You should regularly check your eye pressure while using this medication. This is especially important in children, as the risk of increased ocular pressure caused by corticosteroids may be greater in children and occur earlier than in adults.
- You may also develop cataracts.
Patients with a history of diabetes and myopia are at higher risk.
- You should visit your doctor frequently if you use this medication for a long time.
- Develop Cushing's syndrome due to the medication reaching the bloodstream. Consult your doctor if you experience swelling and weight gain around the trunk and face, as these are usually the first manifestations of Cushing's syndrome. Suppression of adrenal gland function may occur after interrupting intensive or long-term treatment with Colircusí Gentadexa. Consult your doctor before interrupting treatment on your own. These risks are especially important in children and patients treated with a medication called ritonavir or cobicistat.
- Corticosteroids applied to the eye can delay the healing of eye wounds. It is also known that ophthalmic non-steroidal anti-inflammatory drugs (NSAIDs) slow down or delay wound healing. The combined use of NSAIDs and ophthalmic corticosteroids may increase healing problems.
- If you have a disorder that causes thinning of the eye tissues before using this medication, consult your doctor, as it could cause corneal perforation.
- With prolonged use of this medication, you may experience abnormal dryness in the eyes, which can worsen the symptoms of allergic conjunctivitis.
- Contact your doctor if you experience blurred vision or other visual disturbances.
- If you wear contact lenses:
- Wearing contact lenses (hard or soft) is not recommended during the treatment of an eye inflammation or infection.
Otic use
In case of use in the ears (otic route), the doctor should check the condition of your eardrum.
Children
Do not use Colircusí Gentadexa in children under 18 years of age, as its safety and efficacy have not been established.
Elderly patients
The safety and efficacy of this medication have not been established in elderly patients.
Other medications and Colircusí Gentadexa
Inform your doctor or pharmacist if you are using, have recently used, or may need to use any other medication.
Inform your doctor if you are using ophthalmic NSAIDs. The concomitant use of corticosteroids and ophthalmic NSAIDs may increase corneal healing problems.
Especially inform your doctor if you are taking monoamine oxidase inhibitors (MAOIs).
Inform your doctor if you are using ritonavir or cobicistat, as it may increase the amount of dexamethasone in the blood.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Colircusí Gentadexa is not recommended during pregnancy.
If you are breastfeeding, your doctor should decide whether to interrupt breastfeeding or interrupt treatment with this medication, considering the benefit of breastfeeding for the child and the benefit of treatment for the mother.
Driving and using machines
Use in the eyes:You may experience blurred vision immediately after applying Colircusí Gentadexa for a while. Do not drive or use machines until this effect has disappeared.
Use in the ears:No effects on driving or using machines are expected.
Colircusí Gentadexa contains benzalkonium chloride and phosphates
This medication contains 0.04 mg of benzalkonium chloride per ml.
Use in the eyes:Benzalkonium chloride can be absorbed by soft contact lenses, altering their color. Remove contact lenses before using this medication and wait 15 minutes before putting them back on.
Benzalkonium chloride can cause eye irritation, especially if you have dry eye or other corneal diseases (transparent layer of the front of the eye). Consult your doctor if you feel a strange sensation, itching, or pain in the eye after using this medication.
This medication contains 1.8 mg of phosphate per ml.
If you have severe corneal damage (the transparent layer of the front of the eye), treatment with phosphates, in rare cases, can cause blurred vision due to calcium accumulation.
Use in the ears:Benzalkonium chloride can cause skin irritation. Do not apply to the mucous membranes.
3. How to use COLIRCUSÍ GENTADEXA
Follow the administration instructions of this medication indicated by your doctor exactly. In case of doubt, consult your doctor or pharmacist again.
Colircusí Gentadexa can be used as eye drops (ophthalmic route) and ear drops (otic route).
How to use in the eye (ophthalmic route)
The recommended dose is:
Adults: 1 or 2 drops in the affected eye(s) every 4 or 5 hours (in severe infections, the frequency of instillation may be increased). The frequency may be reduced according to your doctor.
Treatment should be gradually reduced, reducing the frequency of administration.
Treatment should not exceed 14 days, unless otherwise indicated by your doctor.
Recommendations for use
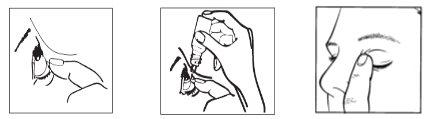
1 2 3
- Wash your hands.
- Take the bottle (dropper container).
- After opening the bottle for the first time, remove the plastic ring from the seal if it is loose.
- Hold the bottle, upside down, between your fingers.
- Tilt your head back. Gently separate the eyelid from the eye with one finger until a pocket forms between the eyelid and your eye, where the drop should fall (figure 1).
- Bring the tip of the bottle close to the eye. You may find it helpful to use a mirror.
- Do not touch the eye or eyelid, nearby areas, or other surfaces with the dropper. The drops may become contaminated.
- Gently squeeze the base of the bottle with your index finger to release one drop at a time (figure 2).
- After using the eye drops, release the eyelid, close your eye, and gently press the edge of the eye next to the nose for at least 2 minutes. This helps prevent the medication from entering the rest of the body (figure 3).
- If you apply drops to both eyes, repeat the above steps for the other eye.
- Close the bottle tightly immediately after using the product.
If a drop falls outside the eye, try again.
If your symptoms do not improve within 2 days, consult your doctor.
If you use more COLIRCUSÍ GENTADEXA than you should, you can eliminate it by washing your eyes with warm water. Do not apply more drops until it is time for your next dose.
If you are using other ophthalmic medications, wait at least 5 minutes between the administration of this eye drop and other ophthalmic medications. Ophthalmic ointments should be administered last.
How to use in the ear
The recommended dose is:
Adults: 3 or 4 drops in the affected ear(s), 3 times a day.
Treatment should not exceed 14 days.
Recommendations for use
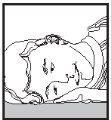
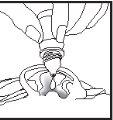
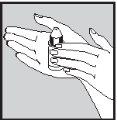
1 2 3
- Wash your hands.
- Take the bottle (dropper container) and hold it in your hand for a few minutes to warm the contents and avoid the drop being too cold when it enters the ear (figure 1).
- Hold the bottle upside down between your fingers.
- Lie on your side with the affected ear facing up.
- Bring the tip of the bottle close to the ear canal (figure 2).
- Do not touch the earlobe, ear canal, or nearby areas or other surfaces with the dropper. The drops may become contaminated.
- Let one drop of this medication fall each time, gently squeezing the base of the bottle with your index finger.
- Remain lying down for at least 5 minutes to allow the ear drops to flow into the ear canal (figure 3).
- If you apply drops to both ears, repeat the above steps for the other ear.
- Close the bottle tightly immediately after using the product.
If a drop falls outside the ear, try again.
If you use more COLIRCUSÍ GENTADEXA than you should
Do not apply more drops until the next dose.
An overdose in the eyes can be eliminated by washing the eyes with warm water.
Severe reactions such as symptoms of central nervous system depression and severe reactions affecting the heart and circulation may occur (especially in children); among other symptoms, decreased body temperature, slow heart rate, sweating, fainting, and stroke.
Other symptoms that may appear if the medication is accidentally ingested, especially by children, are: palpitations, irregular heartbeat, headache, dizziness, nausea, vomiting, dilated pupils, difficulty breathing, or others.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately, or go to a medical center or call the Toxicology Information Service, phone 91 562 04 20, indicating the medication and the amount used.
If you forget to use COLIRCUSÍ GENTADEXA
Do not apply a double dose to make up for the forgotten dose.
Apply a single dose as soon as you remember, and continue with the next scheduled dose. However, if it is almost time for the next dose, do not apply the forgotten dose and continue with the next dose of your regular regimen.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
The following adverse effects have been observed when using this medicine in the eye.
Frequency not known (cannot be estimated from the available data):
- Eye effects: eye irritation, eye pain (including burning), local allergy, blurred vision.
- General adverse effects: allergy (hypersensitivity).
- Hormonal problems: excessive growth of body hair (especially in women), muscle weakness and wasting, purple streaks on the skin of the body, increased blood pressure, irregular or absent menstrual periods, changes in the body's protein and calcium levels, growth delay in children and adolescents, and swelling and weight gain of the body and face (Cushing's syndrome) (see section 2, "Warnings and Precautions").
Rare cases of corneal calcification have been reported with the use of eye drops containing phosphates, in patients with significantly affected corneas.
Description of selected adverse effects
Eye use:
Prolonged use of ophthalmic corticosteroids can cause ocular hypertension and/or glaucoma, which can lead to optic nerve damage, affecting visual acuity and visual field; it can also cause cataracts. And there may be delayed corneal healing.
The corticosteroid (dexamethasone) can cause dizziness, headache, corneal inflammation, conjunctivitis, dry eye, corneal staining, sensitivity to sunlight (photophobia), blurred vision, eye itching, foreign body sensation, increased tearing, eyelid margin crusting, eye redness, corneal erosion, pupil dilation.
In patients with diseases that cause thinning of the cornea or sclera, the topical use of corticosteroids can cause corneal perforation, more likely in prolonged treatments.
Secondary infections have developed after the combined use of corticosteroid and antimicrobial drugs (such as gentamicin).
Due to the presence of tetrizoline in the composition, in addition to other adverse effects mentioned, eyelid inflammation (blepharitis) may appear, as well as systemic adverse effects (in internal parts of the body) as a result of absorption, which seem to be more frequent in children and the elderly. Systemic toxicity has been reported with the local application of sympathomimetic drugs, such as tetrizoline, such as headache, increased blood pressure, sweating, heart rhythm alterations (extrasystoles), rapid heartbeat, fainting, and cerebrovascular accidents.
Other adverse reactions reported via the ophthalmic route are epithelial defects in the conjunctiva and redness of it, conjunctivitis with a membrane-like appearance (pseudomembranous).
Ear use:
Although rare, patients who require prolonged use of aminoglycoside antibiotics via the otic route for the treatment of chronic otitis media have experienced hearing loss that can affect both the inner ear and the auditory nerve (neurosensorial).
Other adverse effects via the otic route are: acne in prolonged administration, delayed healing, and skin irritation.
Additional Adverse Effects in Children
Excessive use of this medicine in children can cause serious reactions such as decreased body temperature, fainting, or other symptoms of central nervous system depression.
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that does not appear in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaRAM.es.By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Conservation of COLIRCUSÍ GENTADEXA
Keep this medicine out of sight and reach of children.
Do not store at a temperature above 30 ºC.
Do not use this medicine after the expiration date that appears on the bottle and on the box after CAD. The expiration date is the last day of the month indicated.
To avoid infections, the bottle must be discarded 4 weeks after it has been opened for the first time.
Write the opening date of the bottle in the box reserved for this purpose.
Medicines should not be thrown away through the sewers or in the trash. Deposit the containers and medicines you no longer need at the SIGRE Point of the pharmacy. In case of doubt, ask your pharmacist how to dispose of the containers and medicines you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Composition of Colircusí Gentadexa
- The active ingredients are gentamicin, sodium dexamethasone phosphate, and tetrizoline hydrochloride. One ml of solution contains 5 mg of gentamicin sulfate (0.5%) (corresponding to 3 mg/ml of gentamicin), 1 mg of sodium dexamethasone phosphate (0.1%), and 0.5 mg of tetrizoline hydrochloride (0.05%).
- The other components are: benzalkonium chloride, disodium hydrogen phosphate dodecahydrate, sodium chloride, povidone, and purified water / water for injectable preparations.
Appearance of the Product and Package Contents
Colircusí Gentadexa is an eye drop (for ophthalmic use) or ear drop (for otic use) in solution; it is a clear, colorless, or slightly yellowish liquid.
It is presented in a dropper bottle (plastic bottle with screw cap), which contains 10 ml of solution.
Marketing Authorization Holder
NTC s.r.l., via Luigi Razza, 3, 20124 Milan, Italy
Manufacturer
SIEGFRIED El Masnou, S.A.
C/ Camil Fabra, 58
08320 El Masnou – Barcelona,
Spain
O
EXCELVISION S.A.
27, Rue de la Lombardière
07100 Annonay
France
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
NTC Ophthalmics Iberica S.L., Calle Pinar, 5, 28006 Madrid, Spain
Date of the Last Revision of this Prospectus:January 2019
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price4.45 EUR
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
Online doctors for COLIRCUSI GENTADEXA 5 mg/mL + 1 mg/mL + 0.5 mg/mL EYE/EAR DROPS SOLUTION
Discuss questions about COLIRCUSI GENTADEXA 5 mg/mL + 1 mg/mL + 0.5 mg/mL EYE/EAR DROPS SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions








