
COLIRCUSI FENILEFRINA 100 mg/ml eye drops solution


How to use COLIRCUSI FENILEFRINA 100 mg/ml eye drops solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
COLIRCUSI PHENYLEPHRINE 100 mg/ml eye drops, solution
Phenylephrine hydrochloride
Read the entire package leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What COLIRCUSI PHENYLEPHRINE is and what it is used for
- What you need to know before using COLIRCUSI PHENYLEPHRINE
- How to use COLIRCUSI PHENYLEPHRINE
- Possible side effects
- Storage of COLIRCUSI PHENYLEPHRINE
- Contents of the pack and further information
1. What COLIRCUSI PHENYLEPHRINE is and what it is used for
It is an eye drop that contains phenylephrine hydrochloride as the active ingredient, which dilates the pupil.
Colircusi Phenylephrine is indicated for: producing dilation of the pupil (mydriasis), to examine the fundus of the eye, to facilitate cataract surgery (nuclear cataract), and as part of the treatment of inflammation of the middle layer of the eye (uveitis), in particular, the colored part of the eye (iritis) and inflammation of this and the ciliary body (iridocyclitis).
2. What you need to know before using COLIRCUSI PHENYLEPHRINE
Do not use Colircusi Phenylephrine:
- If you are allergic to phenylephrine or any of the other components of this medication (listed in section 6).
- In children under 12 years of age and elderly patients.
- If you have narrow-angle glaucoma (increased pressure in the eyes) or narrow angle in your eyes due to anatomy (with blockage of the drainage channels).
- If you have high blood pressure (severe hypertension).
- If you have a history of severe arteriosclerotic disease, cardiovascular or cerebrovascular disease, rapid heartbeats (ventricular tachycardia), or heart disease. If you have any aneurysm (enlargement of a blood vessel that could appear as a lump).
- If you have had eye surgery.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Colircusi Phenylephrine.
- Use this medication only in your eye(s).
- Do not use it after eye surgery, or in diseased, damaged, tearless, or anesthetized eyes, as the medication may be absorbed in sufficient quantity to produce side effects such as increased blood pressure.
- After application, keep your eyes closed while gently pressing the lacrimal canal with your finger for 2 minutes. See section 3, "How to use COLIRCUSI PHENYLEPHRINE".
- Blood pressure should be monitored after application of this medication in susceptible patients, particularly in patients with any disease of the autonomic nervous system (which controls involuntary actions, such as heartbeats, blood vessels, etc.).
- Caution should be exercised or treatment should be avoided when using this medication with medications such as monoamine oxidase inhibitors (MAOIs), tricyclic antidepressants, antihypertensive medications (to lower blood pressure), atropine, or anesthetics that sensitize the heart to sympathomimetics (see "Other medications and Colircusi Phenylephrine").
- Wash your hands immediately after handling the bottle or after applying this medication.
- This medication should be used with caution:
- if you have partial heart block (conduction defect in the heart),
- slow heartbeats (bradycardia),
- if you have excessive thyroid gland activity (hyperthyroidism),
- if you are insulin-dependent diabetic,
- if you experience low blood pressure (fainting, dizziness) related to changes in position (when standing up after sitting for a long time) (orthostatic hypotension).
- Colircusi Phenylephrine should not be used in children under 12 years of age, as children seem to be more sensitive to the risk of serious side effects.
- Colircusi Phenylephrine is not recommended in children between 12 and 18 years of age, as there is a lack of adequate clinical experience.
Children
- This medication should not be used in children under 12 years of age due to the increased risk of systemic toxicity. Children may be especially sensitive to the effects of phenylephrine, with possible serious side effects on the heart and circulation.
Elderly patients
- This medication should not be used in elderly patients. In them, the medication has stimulating effects on the central nervous system. Also, these patients suffer from a greater increase in blood pressure than young people.
- This medication could produce the opposite effect of dilation, i.e., miosis (pupil constriction) the next day. The doctor should monitor this effect.
- In older patients, it is more common for floating pigment granules to occur in the eyes.
Other medications and Colircusi Phenylephrine
Tell your doctor or pharmacist if you are using, have recently used, or may need to use any other medication.
Especially, tell your doctor if you are using or may be exposed to:
- Monoamine oxidase inhibitors (MAOIs). Even if you have taken them during the last 3 weeks (they are medications for treating depression or Parkinson's disease).
- Tricyclic antidepressants.
- Medications that lower blood pressure, called antihypertensives (guanethidine, reserpine, and non-selective beta-blockers such as propranolol).
- Cycloplegic medications (pupil dilators) of the atropine type.
- Inhaled anesthetic medications.
- Methylene blue (used in surgery, among other uses).
Tell your doctor if you are going to undergo surgery with anesthesia.
Use of Colircusi Phenylephrine with food and drinks
- Bitter orange-based beverages: if they contain any stimulant similar to the active ingredient of this medication, an increase in stimulating effects may occur.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
As a precaution, it is preferable to avoid using Colircusi Phenylephrine during pregnancy.
If you are breastfeeding, your doctor should decide whether to interrupt breastfeeding or interrupt treatment with this medication, taking into account the benefit of breastfeeding for the child and the benefit of treatment for the mother.
Driving and using machines
You may experience blurred vision and other visual disturbances for a prolonged period. Do not drive or use machines until this effect has disappeared.
Colircusi Phenylephrine contains thiomersal and phosphates
This medication may cause allergic reactions because it contains thiomersal.
This medication contains 1.1 mg of phosphates per ml.
If you have severe corneal damage (the transparent layer on the front of the eye), treatment with phosphates, in very rare cases, may cause blurred vision due to calcium accumulation.
3. How to use COLIRCUSI PHENYLEPHRINE
Follow the instructions for administration of this medication exactly as indicated by your doctor. If you are in doubt, consult your doctor or pharmacist again.
- To limit the amount of medication that passes into the bloodstream after application of the eye drops
keep your eyes closed while gently pressing the lacrimal canal with your finger for at least 2 minutes.
The recommended dose is:
Use in adults
For examination of the fundus of the eye, it is recommended to apply one drop in each eye. If necessary, the application can be repeated in 1 hour.
When a sustained effect is sought, it is recommended to apply one drop 2 or 3 times a day.
Before cataract surgery, the usual dose is one drop in the conjunctiva 30 to 60 minutes before surgery.
In the treatment of iritis and iridocyclitis, apply one drop. If necessary, it can be repeated the next day. Treatment should not exceed 3-5 consecutive days; if there is no improvement, consult your doctor.
Use in children
This medication is contraindicated in children under 12 years of age due to the increased risk of systemic toxicity.
Recommendations for use:
12
- Wash your hands.
- Take the bottle.
- Remove the seal by pulling the tab outward and remove the cap.
- Hold the bottle upside down between your fingers.
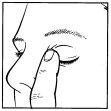
- Tilt your head back. Gently separate the lower eyelid from your eye until a pouch forms between the lower eyelid and your eye, where the drop should fall (figure 1).
- Place the tip of the dropper close to your eye. You may find it helpful to use a mirror.
- Do not touch your eye, eyelid, or surrounding areas with the dropper. The eye drops may become contaminated.
- Gently squeeze the rubber dropper located at the top of the bottle while looking up.
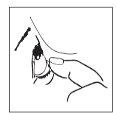
- After using this eye drop, press the edge of your eye near your nose with your finger for at least 2 minutes (figure 2). This helps prevent the medication from passing into the rest of your body.
- If you are applying drops in both eyes, repeat all the previous steps with the other eye.
- Close the bottle tightly immediately after use.
- It is recommended to wash your hands after handling the bottle or after applying the eye drops.
If a drop falls outside the eye, try again.
If you are using other eye medications, wait at least 5 minutes between administration of this medication and other eye medications. Ophthalmic ointments should be administered last.
If you use more COLIRCUSI PHENYLEPHRINE than you should
You can eliminate it by washing your eyes with lukewarm water. Do not apply more drops until it is time for your next dose.
In case of overdose or accidental ingestion, go to a medical center or consult your doctor or pharmacist immediately, or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medication and the amount used.
The symptoms of toxicity that may appear are due to sympathetic stimulation. In case of accidental ingestion, this medication can cause high blood pressure, headache, convulsions, cerebral hemorrhage, abnormal heart palpitations, sensations of tingling, heat, or cold in the skin (paresthesia), or vomiting. Fluid in the lungs (pulmonary edema) and cardiac arrest.
In case of overdose and because any severe reaction due to phenylephrine appears rapidly, it is recommended to go to a medical center for maintenance treatment.
For the treatment of hypertension as a consequence of vasoconstriction, the use of beta-blockers and calcium channel blockers should be avoided.
If you forget to use COLIRCUSI PHENYLEPHRINE
Do not apply a double dose to make up for forgotten doses.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
The following side effects have been reported with this medication:
Side effects of unknown frequency (cannot be estimated from available data).
- Eye effects: eye pain, eye irritation, eye redness, conjunctival inflammation, and/or eczema on the eyelid.
- General effects: allergy, dizziness, increased blood pressure, increased heart rate (heartbeats), fluid in the lungs, skin inflammation.
Other side effects in children
Fluid or swelling in the lungs: unknown frequency (cannot be estimated from available data).
Other side effects that may be caused by this type of medication
In very rare cases, some patients with severely damaged cornea (the transparent layer on the front of the eye) have experienced cloudy areas in the cornea due to calcium accumulation during treatment.
Reported are: headache, heart rhythm disorders (extrasystoles), fainting. The incidence of adverse events with phenylephrine 100 mg/ml is higher than with lower concentrations.
This eye drop has occasionally caused serious cardiovascular complications, including myocardial infarction.
Reporting of side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medications: https://www.notificaRAM.es.By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of COLIRCUSI PHENYLEPHRINE
Keep this medication out of the sight and reach of children.
Before the first opening, store in the refrigerator (between 2°C and 8°C).
After opening, do not store above 25°C.
To avoid infections, the bottle should be discarded 4 weeks after it was first opened.
Write the date of opening of the bottle in the space provided for this purpose on the carton.
Do not use this medication after the expiration date stated on the bottle and carton after EXP. The expiration date is the last day of the month indicated.
Medications should not be disposed of via wastewater or household waste. Place the packaging and any unused medication in the SIGRE collection point at your pharmacy. If you have any questions, ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
6. Contents of the pack and further information
Composition of COLIRCUSI PHENYLEPHRINE
- The active ingredient is phenylephrine hydrochloride. One ml of solution contains 100 mg of phenylephrine hydrochloride (10%).
- The other ingredients are: thiomersal, disodium hydrogen phosphate dodecahydrate, anhydrous sodium sulfite, and purified water.
Appearance of the product and contents of the pack
Colircusi Phenylephrine is an eye drop solution; it is a clear, colorless, or slightly yellowish liquid.
It is presented in a dropper bottle (opaque glass bottle with a rubber dropper), containing 10 ml of eye drop solution.
Marketing authorization holder
M4 PHARMA, S.L.
Tánger, 86
08018 Barcelona – Spain
Manufacturer
TUBILUX PHARMA, S.P.A.
Via Costarica 20/22
Pomezia, Rome - I-00071
Italy
Date of last revision of this package leaflet:January 2021
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Average pharmacy price5.34 EUR
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to COLIRCUSI FENILEFRINA 100 mg/ml eye drops solutionDosage form: EYEDROP, 100 mg/mlActive substance: phenylephrineManufacturer: Bausch + Lomb Ireland LimitedPrescription requiredDosage form: EYEDROP, -Active substance: atropineManufacturer: Alcon Healthcare S.A.Prescription requiredDosage form: EYEDROP, 5 mgActive substance: atropineManufacturer: Alcon Healthcare S.A.Prescription required
Online doctors for COLIRCUSI FENILEFRINA 100 mg/ml eye drops solution
Discuss questions about COLIRCUSI FENILEFRINA 100 mg/ml eye drops solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















