CLINIMIX N9G15E SOLUTION FOR INFUSION

How to use CLINIMIX N9G15E SOLUTION FOR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Patient Information: Summary of Product Characteristics
Clinimix N9G15E solution for infusion
Read this entire leaflet carefully before starting treatment with this medication, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or nurse.
- If you experience side effects, consult your doctor or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the leaflet:
- What Clinimix is and what it is used for
- What you need to know before taking Clinimix
- How Clinimix is administered
- Possible side effects
- Storage of Clinimix
- Package contents and additional information
1. What Clinimix is and what it is used for
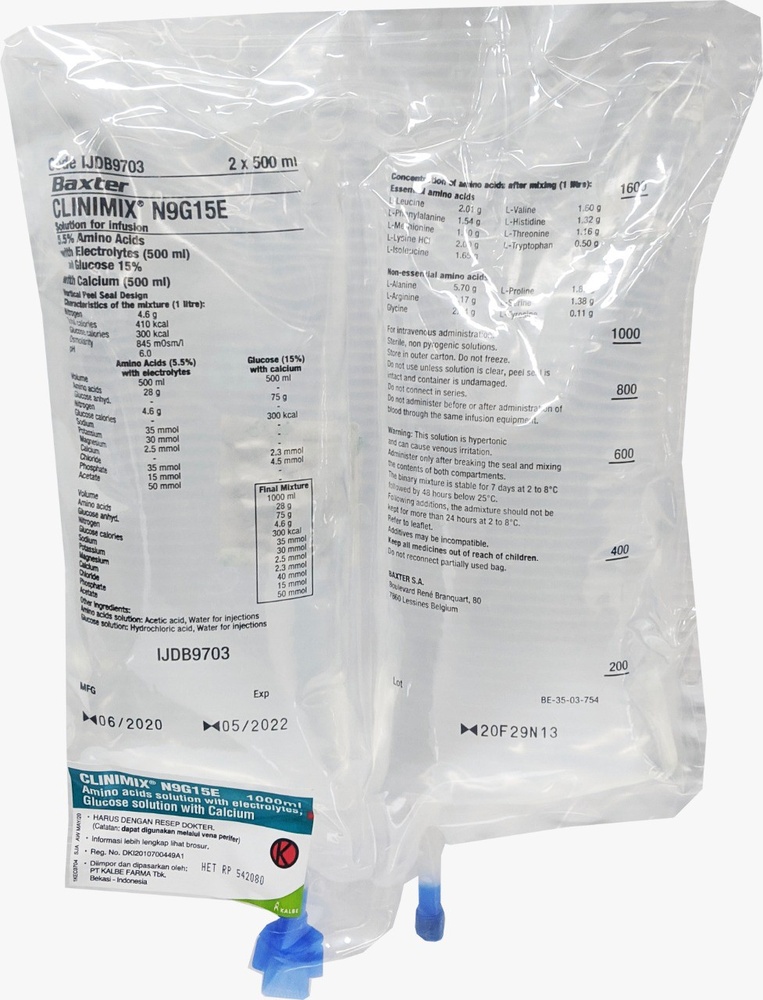
Clinimix is a solution for infusion. It is supplied in a bag with 2 chambers. One chamber contains an amino acid solution with electrolytes, and the second chamber contains a glucose solution with calcium chloride. The chambers are separated by a non-permanent seal. The contents of the chambers must be mixed immediately before administration by rolling the top of the bag to break the seals.
Clinimix is administered to feed adults and children through a tube connected to a vein when normal oral feeding is not suitable.
Clinimix should only be administered under medical supervision.
2. What you need to know before taking Clinimix
Clinimix must not be administered if:
- you are allergic to the active substances or to any of the other components of this medication (listed in section 6),
- your body has problems using certain amino acids,
- you have too much sugar in your blood (severe hyperglycemia),
- your blood is excessively acidic (metabolic acidosis due to excess lactate),
- you have high levels of sodium, potassium, magnesium, calcium, and/or phosphorus in your blood (hypernatremia, hyperkalemia, hypermagnesemia, hypercalcemia, and/or hyperphosphatemia),
- in children under 28 days, ceftriaxone must not be co-administered with intravenous solutions containing calcium, as particles may form.
In all cases, your doctor will decide whether you should be given this medication based on factors such as age, weight, and clinical condition, along with the results of all tests performed.
Warnings and precautions
Consult your doctor or nurse before Clinimix is administered to you.
If any abnormal signs or symptoms of an allergic reaction occur, such as fever, chills, skin rash, or difficulty breathing, excessive sweating, nausea, and headache, tell your doctor or nurse: the infusion will be stopped immediately. Your doctor will monitor your condition while you are being given this medication and may change the dose or add other nutrients, such as lipids, vitamins, electrolytes, and trace elements if necessary.
Certain medications and diseases may increase the risk of developing an infection or sepsis (bacteria in the blood). There is a special risk of infection or sepsis when a tube (intravenous catheter) is inserted into your vein. Your doctor will closely monitor you for any signs of infection. The use of aseptic techniques (germ-free) when inserting and maintaining the catheter and preparing the nutritional formula can reduce the risk of infection.
Clinimix with electrolytes contains calcium. It must not be administered with the antibiotic ceftriaxone, as particles may form.
If you are severely malnourished and require nutrition through a vein, it is recommended that parenteral nutrition be started slowly and with caution.
Your doctor will monitor your condition at the start of the infusion, especially if you currently have liver, kidney, adrenal, heart, or circulation problems. Your doctor should also be aware of severe conditions that affect how your body handles sugars, fats, proteins, or salts (metabolic disorders). If any abnormal signs occur, including venous irritation, the infusion should be stopped.
To check the effectiveness and safety of the administration, your doctor will perform laboratory and clinical tests while you are being given this medication. If you are given this medication for several weeks, your blood will be regularly analyzed. In particular, in cases of glucose intolerance, blood glucose and urine glucose will be routinely monitored, and if you are a diabetic patient, the insulin dose may need to be adjusted.
Children and adolescents
When used in newborns and children under 2 years, the solution (in the bags and administration equipment) must be protected from light exposure until administration is complete. Exposure of Clinimix to ambient light, especially after mixing with trace elements and/or vitamins, generates peroxides and other degradation products that can be reduced by protecting the solution from light.
Interaction of Clinimix with other medications
Tell your doctor or nurse if you are taking or have recently taken or may need to take any other medication.
Clinimix with electrolytes contains calcium. It must not be administered with the antibiotic ceftriaxone, as particles may form.
Due to the potassium content of Clinimix, special attention should be paid to patients treated with potassium-sparing diuretics (e.g., amiloride, spironolactone, triamterene), angiotensin-converting enzyme inhibitors (ACE inhibitors), angiotensin II receptor antagonists, or the immunosuppressants tacrolimus or cyclosporine, due to the risk of hyperkalemia.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, or think you may be pregnant or plan to become pregnant, consult your doctor before using this medication.
3. How Clinimix is administered
Before administering the product, the non-permanent seal between the two compartments must be broken and the contents of both mixed.
Clinimix can be administered to adults and children.
This is a solution for infusion that is administered through a plastic tube connected to a vein in your arm or a large vein in your chest.
When used in newborns and children under 2 years, the solution (in the bags and administration equipment) must be protected from light exposure until administration is complete (see section 2).
Dose – Adults and children
Your doctor will decide the dose you need and the duration of administration, based on your age, weight, height, clinical condition, daily fluid volume, and energy and nitrogen needs.
Follow your doctor's instructions for administering Clinimix exactly. Consult your doctor if you have any questions.
Administration can continue for as long as necessary, depending on your clinical condition.
The infusion of one bag usually lasts between 8 and 24 hours.
If you are given too much Clinimix
If the administered dose is too high or the infusion too rapid, it can cause an increase in your blood circulation volume or make your blood too acidic. The glucose content can increase your blood and urine glucose levels. Excessive administration can cause nausea, vomiting, tremors, and electrolyte disturbances. In these situations, the infusion must be stopped immediately.
In some severe cases, your doctor may need to perform temporary renal dialysis to help your kidneys eliminate excess product.
To avoid these situations, your doctor will regularly monitor your condition and analyze your blood parameters.
If you have any further questions about the use of this product, ask your doctor.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone gets them. If you notice any change in the way you feel during or after treatment, tell your doctor or nurse immediately.
The tests your doctor will perform while you are being given this medication should minimize the risk of side effects.
The infusion will be stopped immediately if any abnormal signs or symptoms of an allergic reaction occur, such as abnormally high or low blood pressure, blue or purple discoloration of the skin, abnormally high heart rate, difficulty breathing, vomiting, nausea, skin rash, increased body temperature, excessive sweating, and chills.
Other side effects have been observed, which occur more or less frequently:
- Anaphylaxis (a severe allergic reaction that can be life-threatening).
- High levels of glucose, ammonia, and nitrogen-containing compounds in the blood.
- Impaired liver function, abnormal liver function blood tests.
- Inflammation of the gallbladder, presence of gallstones in the gallbladder.
- Inflammation of the veins at the infusion site, venous irritation, pain, irritation, burning, swelling.
- Presence of glucose in the urine.
- Diabetic coma.
- Formation of small particles that block the pulmonary blood vessels.
Reporting side effects:
If you experience any side effects, consult your doctor or nurse, even if they are not listed in this leaflet. You can also report them directly through the Spanish Medicines Monitoring System: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Clinimix
Keep this medication out of the sight and reach of children.
When used in newborns and children under 2 years, the solution (in the bags and administration equipment) must be protected from light exposure until administration is complete (see section 2).
Do not use this medication after the expiration date stated on the packaging and outer packaging (MM/YYYY). The expiration date is the last day of the month indicated.
Do not freeze.
Keep the container in the outer packaging.
Do not dispose of medications through wastewater or household waste. If in doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This will help protect the environment.
6. Container Content and Additional Information
Clinimix Composition
The active principles of each bag of the reconstituted solution are:
Active Principles | 1 l | 1.5 l | 2 l |
L-alanine | 5.70 g | 8.54 g | 11.39 g |
L-arginine | 3.17 g | 4.75 g | 6.33 g |
Glycine | 2.84 g | 4.25 g | 5.67 g |
L-histidine | 1.32 g | 1.98 g | 2.64 g |
L-isoleucine | 1.65 g | 2.48 g | 3.30 g |
L-leucine | 2.01 g | 3.02 g | 4.02 g |
L-lysine (as lysine hydrochloride) | 1.60 g (2.00 g) | 2.39 g (2.99 g) | 3.19 g (3.99 g) |
L-methionine | 1.10 g | 1.65 g | 2.20 g |
L-phenylalanine | 1.54 g | 2.31 g | 3.08 g |
L-proline | 1.87 g | 2.81 g | 3.74 g |
L-serine | 1.38 g | 2.06 g | 2.75 g |
L-threonine | 1.16 g | 1.73 g | 2.31 g |
L-tryptophan | 0.50 g | 0.74 g | 0.99 g |
L-tyrosine | 0.11 g | 0.17 g | 0.22 g |
L-valine | 1.60 g | 2.39 g | 3.19 g |
Sodium acetate 3H2O | 2.16 g | 3.23 g | 4.31 g |
Dibasic potassium phosphate | 2.61 g | 3.92 g | 5.22 g |
Sodium chloride | 1.12 g | 1.68 g | 2.24 g |
Magnesium chloride 6H2O | 0.51 g | 0.77 g | 1.02 g |
Anhydrous glucose (as glucose monohydrate) | 75 g (83 g) | 113 g (124 g) | 150 g (165 g) |
Calcium chloride 2H2O | 0.33 g | 0.50 g | 0.66 g |
The other components are:
- acetic acid, hydrochloric acid (for pH adjustment of the solution),
- water for injectable preparations.
Appearance of CLINIMIX and Container Content
CLINIMIX is an infusion solution presented in a multi-layer plastic bag with two chambers. The material of the inner layer (in contact) of the bag is made of polymers (a mixture of polyolefin copolymers) to be compatible with the components and authorized additives. Other layers are made of EVA (polyethylene-vinyl acetate) and a copolyester.
Before reconstitution, the glucose and amino acid solutions are transparent, colorless, or slightly yellowish. After reconstitution, the solution is also transparent, colorless, or slightly yellowish.
To avoid contact with air oxygen, the bag is packaged inside an overbag that acts as an oxygen barrier, which contains an oxygen absorber.
Container Sizes
1000 ml bag: cardboard box with 8 bags
1 bag of 1000 ml
1500 ml bag: cardboard box with 6 bags
1 bag of 1500 ml
2000 ml bag: cardboard box with 4 bags
1 bag of 2000 ml
Only some container sizes may be marketed.
Marketing Authorization Holder
Baxter S.L.
Pouet de Camilo 2,
46394 Ribarroja del Turia (Valencia) Spain
Manufacturer
Baxter SA, Boulevard René Branquart, 80, 7860 Lessines, Belgium
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Clinimix N9G15E, solution for infusion
In some countries, it is registered under a different name, as indicated below
Germany: Clinimix 3% GE
The last revision of this leaflet was in September 2021
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
-------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
- Quantitative composition
After mixing the contents of the two compartments, the composition of the binary mixture for all available bag sizes provides the following:
1 l | 1.5 l | 2 l | |
Nitrogen (g) Amino acids (g) Glucose (g) | 4.6 28 75 | 6.8 41 113 | 9.1 55 150 |
Total calories (kcal) Glucose calories (kcal) | 410 300 | 615 450 | 820 600 |
Sodium (mmol) Potassium (mmol) Magnesium (mmol) Calcium (mmol) | 35 30 2.5 2.3 | 53 45 3.8 3.4 | 70 60 5.0 4.5 |
Acetate (mmol) Chloride (mmol) Phosphate as HPO4 2- (mmol) | 50 40 15 | 75 60 23 | 100 80 30 |
pH Osmolality (mOsm/l) | 6 845 |
- Dosage and Administration
Before administering the product, the non-permanent seal between the two compartments must be broken and the contents of both mixed.
Dose and Infusion Rate
The dose should be individualized according to the patient's nutritional/liquid needs, energy expenditure, clinical status, body weight, and ability to metabolize Clinimix components, as well as any additional energy or proteins administered orally/enterally. Additionally, daily fluid, nitrogen, and energy needs continuously decrease with age.
In adults, needs range from 0.16 g of nitrogen/kg/day (approximately 1 g of amino acid/kg/day) to 0.32 g of nitrogen/kg/day (approximately 2 g of amino acid/kg/day).
In infants, needs range from 0.16 g of nitrogen/kg/day (approximately 1 g of amino acid/kg/day) to 0.40 g of nitrogen/kg/day (approximately 2.5 g of amino acid/kg/day).
In adults and patients 12 to 18 years old, calorie needs range from 25 kcal/kg/day to 40 kcal/kg/day, depending on the patient's nutritional status and level of catabolism. Patients under 12 years old may have higher requirements.
There may be clinical situations where patients require amounts of nutrients that differ from the composition of Clinimix. In this situation, any volume adjustment (dose) should take into account the resulting effect on the dosing of all other nutritional components of Clinimix. The rate and volume of infusion should be established by a prescribing physician with experience in pediatric intravenous fluid therapy.
This medicinal product does not contain the amino acids cysteine and taurine, considered conditionally essential for neonates and infants.
This medicinal product is not recommended for premature, full-term newborns, and children under 2 years old.
The administration rate should be adjusted based on the dose, characteristics of the infused solution, total volume intake in 24 hours, and duration of infusion.
The infusion time should be more than 8 hours. Normally, the administration rate is gradually increased during the first hour without exceeding 3 ml per kilogram of body weight per hour, and the maximum dose is 40 ml per kilogram of body weight per day.
Administration Method
When used in newborns and children under 2 years old, the solution (in bags and administration equipment) should be protected from light exposure until administration is completed.
Route of Administration
It will be administered by peripheral or central intravenous route depending on the final osmolality of the mixture. In general, the accepted limit for peripheral infusion is approximately 800 mOsm/l, but it varies considerably depending on age, patient's general condition, and characteristics of peripheral veins.
- Special Warnings and Precautions for Use
WARNINGS
With CLINIMIX formulations, hypersensitivity/perfusion reactions have been reported, including hypotension, hypertension, peripheral cyanosis, tachycardia, dyspnea, vomiting, nausea, urticaria, skin rash, pruritus, erythema, hyperhidrosis, fever, and chills.
Anaphylaxis has been reported with other parenteral nutrition products.
Special clinical monitoring is necessary when starting any intravenous infusion. If any abnormal sign or symptom occurs, for example, a hypersensitivity reaction or infusion reaction, the infusion should be interrupted immediately.
Solutions containing glucose should be used with caution in patients with known allergy to corn or corn-derived products.
Vascular pulmonary precipitates have been reported in patients receiving parenteral nutrition.
In some cases, fatal outcomes have occurred. Excessive addition of calcium and phosphate increases the risk of calcium phosphate precipitate formation. Precipitates have been reported even in the absence of phosphate salt in the solution. Distal precipitation in the in-line filter has also been reported and is suspected in vivo precipitate formation.
If signs of pulmonary distress occur, the infusion should be interrupted and a medical evaluation initiated.
In addition to solution inspection, infusion equipment and catheters should also be periodically checked for precipitates.
In patients over 28 days old (including adults), ceftriaxone should not be administered intravenously at the same time as solutions containing calcium, including CLINIMIX N9G15E, through the same infusion line. If the same infusion line is used for sequential administration, it should be carefully flushed with a compatible liquid between infusions.
The use of intravenous catheters to administer parenteral formulations, poor catheter maintenance, or contaminated solutions can lead to infection and sepsis.
Immunosuppression and other factors, such as hyperglycemia, malnutrition, and/or underlying disease, can predispose patients to infectious complications.
Symptomatic care and laboratory control of fever/chills, leukocytosis, technical complications with the access device, and hyperglycemia can help recognize early infections.
The occurrence of septic complications can be reduced by placing greater emphasis on the use of aseptic technique in catheter placement, maintenance, and preparation of the nutritional formula.
Re-feeding of severely malnourished patients can lead to refeeding syndrome, characterized by changes in potassium, phosphorus, and magnesium intracellularly as the patient becomes anabolic. Thiamine deficiency and fluid retention may also occur. Strict supervision and gradual intake of nutrients, avoiding overfeeding, can prevent these complications.
Hypertonic solutions can cause venous irritation if infused through a peripheral vein. The choice of a peripheral or central vein depends on the final osmolality of the mixture.
The generally accepted limit for peripheral infusion is around 800 mOsm/l, but it varies considerably with age and the patient's general condition and characteristics of peripheral veins.
Do not connect plastic containers in series to avoid gas embolisms due to possible residual air contained in the primary container.
PRECAUTIONS
Before starting the infusion, severe alterations in water and electrolyte balance, severe fluid overload, and severe metabolic disorders should be corrected.
Metabolic complications can occur if nutrient intake is not adapted to the patient's requirements or the metabolic capacity of any food component is not accurately assessed. Adverse metabolic effects can occur due to inadequate or excessive administration of nutrients or an inappropriate mixture composition for the patient's specific needs.
Frequent clinical evaluations and laboratory determinations are essential for proper control during administration. These will include ionogram determination and kidney and liver function tests.
Electrolyte needs of patients receiving these solutions should be carefully determined and controlled, especially in the case of solutions without electrolytes.
Glucose intolerance is a common metabolic complication in severely stressed patients. Infusion of this solution can cause hyperglycemia, glucosuria, and hyperosmolar syndrome. Blood and urine glucose should be routinely controlled, and if necessary, insulin dose should be adjusted for diabetics.
Use with caution in patients with renal insufficiency, especially if hyperpotasemia is present, due to the risk of metabolic acidosis and hyperazotemia if extra-renal waste elimination is not performed. The state of fluids and electrolytes should be carefully controlled in these patients. In case of severe renal insufficiency, specially formulated amino acid solutions should be chosen.
Cautious administration of Clinimix is recommended in patients with adrenal insufficiency.
Circulatory overload should be avoided, especially in patients with pulmonary edema, insufficiency, and/or heart failure. The state of fluids should be carefully controlled.
In addition to routine liver function tests, in patients with pre-existing liver disease or liver insufficiency, possible symptoms of hyperammonemia should be controlled.
It is known that in some patients with parenteral nutrition, hepatobiliary disorders, including cholestasis, hepatic steatosis, fibrosis, and cirrhosis, can occur, leading to liver failure, as well as cholecystitis and cholelithiasis. The etiology of these disorders is believed to be multifactorial and may differ between patients. Those who develop abnormal laboratory parameters or other signs of hepatobiliary disorders should be rapidly evaluated by a clinical expert in liver diseases to identify possible causal and contributing factors and potential therapeutic and prophylactic interventions.
In patients receiving amino acid solutions, an increase in blood ammonia levels and hyperammonemia can occur. In some patients, this may indicate the presence of a congenital disorder of amino acid metabolism (see Section 4.3 of the Summary of Product Characteristics) or liver insufficiency.
Blood ammonia should be frequently measured in newborns and infants to detect hyperammonemia, which may indicate the presence of a congenital abnormality of amino acid metabolism.
Depending on the degree and etiology, hyperammonemia may require immediate intervention.
Too rapid infusion of amino acids can cause nausea, vomiting, and chills. In these cases, the infusion should be immediately interrupted.
Generally, the dose for elderly patients should be cautious, taking into account the higher frequency of liver, kidney, or heart insufficiency and concomitant diseases or pharmacotherapy.
Pediatric Population
- No studies have been conducted in the pediatric population.
- See above regarding monitoring of hyperammonemia in pediatric patients.
Exposure of parenteral nutrition solutions for intravenous use, especially after mixing with trace elements or vitamins, can have adverse effects on the clinical outcome of newborns due to the generation of peroxides and other degradation products. When used in newborns and children under 2 years old, Clinimix should be protected from ambient light until administration is completed.
- Practical Information on Preparation and Handling
Caution: Administer the product only after breaking the seal and mixing the contents of the two compartments
1. |
| 2. |
| 3. |
|
Break from the top to open the overbag. | Remove the front part of the overbag to access the Clinimix bag. Discard the overbag and the packet with the oxygen absorber. | Place the bag on a horizontal and clean surface with the handle in front of you. | |||
4. |
| 5. |
| 6. |
|
Lift the hanger area to remove the solution from the top of the bag. Firmly roll the bag until it opens completely. |
Completely break the seal (approximately halfway). | Mix the contents by inverting the bag at least 3 times. | Hang the bag. Turn the protector to remove it from the administration port. Firmly connect the spike connector. |
Use the solution only if it is transparent, colorless, or slightly yellowish, and if the container is not damaged.
CLINIMIX should be at room temperature before use.
The activation of CLINIMIX can be performed in the overbag or once it has been removed.
For single use.
Do not store partially used containers and discard all equipment after use.
Do not reconnect a partially used bag.
Do not connect in series.
When used in newborns and children under 2 years, it should be protected from light exposure until administration is completed. Exposure of Clinimix to ambient light, especially after mixing with oligoelements or vitamins, generates peroxides and other degradation products that can be reduced if the product is protected from light exposure.
Supplementation
Lipids, vitamins, and oligoelements should be provided to patients receiving parenteral nutrition for a long period.
If the administration of additives is necessary, their compatibility and the stability of the mixtures should be checked.
Supplementation can be done after opening the non-permanent seals for all additives (once the two solutions have been mixed). CLINIMIX can be supplemented with:
- Lipid emulsions (e.g., ClinOleic) at a rate of 50 to 250 ml per liter of CLINIMIX
CLINIMIX N9G15E 1 l + 100 ml of Lipids 20%* | CLINIMIX N9G15E 1.5 l + 100 ml of Lipids 20%* | CLINIMIX N9G15E 2 l + 250 ml of Lipids 20%* | |
Nitrogen (g) Amino acids (g) Glucose (g) Lipids (g) | 4.6 28 75 20 | 6.8 41 113 20 | 9.1 55 150 50 |
Total calories (kcal) Glucose calories (kcal) Lipid calories (kcal) Glucose/lipid ratio | 610 300 200 60/40 | 815 450 200 69/31 | 1320 600 500 55/45 |
Sodium (mmol) Potassium (mmol) Magnesium (mmol) Calcium (mmol) Acetate (mmol) Chloride (mmol) Phosphate as HPO4 2- (mmol) | 35 30 2.5 2.3 50 40 15 | 53 45 3.8 3.4 75 60 23 | 70 60 5.0 4.5 100 80 30 |
pH Osmolality (mOsm/l) | 6 795 | 6 810 | 6 785 |
- Electrolytes: per liter of CLINIMIX
Up to a final concentration of | Sodium | Potassium | Magnesium | Calcium |
80 mmol | 60 mmol | 5.6 mmol | 3.0 mmol |
- Oligoelements: per liter of CLINIMIX
Up to a final concentration of | Copper | 10 μmol | Zinc | 77 μmol |
Chromium | 0.14 μmol | Manganese | 2.5 μmol | |
Fluorine | 38 μmol | Cobalt | 0.0125 μmol | |
Selenium | 0.44 μmol | Molybdenum | 0.13 μmol | |
Iodine | 0.5 μmol | Iron | 10 μmol |
- Vitamins: per liter of CLINIMIX
Up to a final concentration of | Vitamin A | 1750 IU | Biotin | 35 μg |
Vitamin B6 | 2.27 mg | Vitamin B1 | 1.76 mg | |
Vitamin D | 110 IU | Folic acid | 207 μg | |
Vitamin B12 | 3.0 μg | Vitamin B2 | 2.07 mg | |
Vitamin E | 5.1 mg | Vitamin C | 63 mg | |
Vitamin PP | 23 mg | Vitamin B5 | 8.63 mg | |
Vitamin K | 75 μg |
Stability data for the supplementation of CLINIMIX with other commercially available lipid emulsions and other additives or nutrients are available upon request.
If a slight cream formation is observed, mix the mixture completely by gentle agitation to obtain a uniform emulsion before perfusion.
Additions should be made under aseptic conditions.
Additions can be made with a syringe or transfer equipment.
- Addition with syringe or transfer equipment with needle.
- Prepare the injection port (the single tube, see figure 1).
- Puncture the tube and inject.
- Mix the solution and additives.
Incompatibilities
Additives may be incompatible; consult the manufacturer for more details.
If it is necessary to add additives, their compatibility and the stability of the mixtures should be checked.
The solution should not be administered with, before, or after a blood transfusion through the same equipment, given the possibility of pseudoagglutination.
CLINIMIX N9G15E contains calcium ions, which poses an additional risk of coagulation in anticoagulated/preserved blood with citrate or its components.
As with any parenteral nutrition mixture, the proportions of calcium and phosphate should be taken into account. The addition of excess calcium and phosphate, especially in the form of mineral salts, can lead to the formation of calcium phosphate precipitates.
As with other infusion solutions containing calcium, the concomitant administration of ceftriaxone and CLINIMIX N9G15E is contraindicated in newborns (≤ 28 days of age), even if separate infusion lines are used (risk of fatal precipitation of calcium ceftriaxone in the newborn's bloodstream).
In patients over 28 days (including adults), ceftriaxone should not be administered intravenously at the same time as solutions containing calcium, including CLINIMIX N9G15E solutions, through the same infusion line (see Warnings section).
If the same infusion line is used for sequential administration, it should be carefully flushed with a compatible liquid between infusions.
- Validity period
2 years if stored in the overbag.
It is recommended to use the product immediately after opening the non-permanent seal between the 2 chambers. However, once reconstituted (i.e., after opening the internal non-permanent seal), the stability of the reconstituted solution has been demonstrated for a maximum of 7 days at a temperature between 2 °C and 8 °C, followed by a maximum of 48 hours at a temperature not exceeding 25 °C.
From a microbiological point of view, mixtures should be used immediately after additions are made. If not used immediately, the storage time and conditions before use are the responsibility of the user and should not exceed 24 hours at 2-8°C, unless the additions were made under controlled and validated aseptic conditions. If longer storage periods are required under exceptional conditions, the company can be contacted, as it has data on physical and chemical stability in use for 7 days at 2-8°C, followed by 48 hours at a temperature below 25°C, for the products indicated in the previous section.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CLINIMIX N9G15E SOLUTION FOR INFUSIONDosage form: INJECTABLE PERFUSION, 3.92 g / 1.26 g / 7.21 g / 3.36 g / 4.2 g / 5.11 g / 2.94 g / 2.8 g / 4.76 g / 5.07 g / 4.06 g / 14.49 g / 0.28 g / 8.05 g / 3.5 g / 200 gActive substance: combinationsManufacturer: Baxter S.L.Prescription requiredDosage form: INJECTABLE INFUSION, 3.5 g / 200 g / 5.22 g / 1.88 g / 3.92 g / 1.26 g / 7.21 g / 3.36 g / 4.2 g / 5.11 g / 2.94 g / 2.8 g / 662 mg / 1.02 g / 4.76 g / 5.15 g / 5.07 g / 4.06 g / 14.49 g / 0.28 g / 8.05 gActive substance: combinationsManufacturer: Baxter S.L.Prescription requiredDosage form: INJECTABLE PERFUSION, 4.25 g / 300 g / 5.22 g / 1.54 g / 4.76 g / 1.53 g / 8.76 g / 4.08 g / 5.1 g / 6.2 g / 3.57 g / 3.4 g / 662 mg / 1.02 g / 5.78 g / 5.94 g / 6.16 g / 4.93 g / 17.6 g / 0.34 g / 9.78 gActive substance: combinationsManufacturer: Baxter S.L.Prescription required
Online doctors for CLINIMIX N9G15E SOLUTION FOR INFUSION
Discuss questions about CLINIMIX N9G15E SOLUTION FOR INFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions