
CIPROXINA 2 mg/ml + 10 mg/ml OTIC SUSPENSION DROPS

How to use CIPROXINA 2 mg/ml + 10 mg/ml OTIC SUSPENSION DROPS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is CIPROXINA and what is it used for
- CIPROXINA contains two active substances, a steroid anti-inflammatory (hydrocortisone) and an antibiotic (ciprofloxacin); ciprofloxacin is an antibacterial drug of the group known as fluoroquinolones that acts by eliminating the bacteria that cause infections.
- What you need to know before you use CIPROXINA
- How to use CIPROXINA
- Possible side effects
- Storage of CIPROXINA
- Contents of the pack and further information
Introduction
Package Leaflet: Information for the User
CIPROXINA 2 mg/ml + 10 mg/ml Ear Drops Suspension
ciprofloxacin / hydrocortisone
Read this package leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is CIPROXINA and what is it used for
- What you need to know before you use CIPROXINA
- How to use CIPROXINA
- Possible side effects
- Storage of CIPROXINA
- Contents of the pack and further information
1. What is CIPROXINA and what is it used for
CIPROXINA contains two active substances, a steroid anti-inflammatory (hydrocortisone) and an antibiotic (ciprofloxacin); ciprofloxacin is an antibacterial drug of the group known as fluoroquinolones that acts by eliminating the bacteria that cause infections.
One of the active substances of this medicine is an antibiotic. Antibiotics are used to treat bacterial infections and are not effective against viral infections.
It is important that you follow the instructions regarding the dose, administration interval, and treatment duration indicated by your doctor.
Do not store or reuse this medicine. If you have any leftover antibiotic after finishing the treatment, return it to the pharmacy for proper disposal. Do not throw away medicines via wastewater or household waste.
This medicine is used for the treatment of acute external otitis (infection of the ear canal) with an intact eardrum, in adults and children over 2 years of age.
2. What you need to know before you use CIPROXINA
Before prescribing this medicine, your doctor will have examined your eardrums to check that they are not perforated.
Do not use CIPROXINA
- if you are allergic (hypersensitive) to ciprofloxacin or to other medicines of the fluoroquinolone group.
- if you are allergic (hypersensitive) to hydrocortisone or to any of the other ingredients included in section 6.
- if your eardrum is perforated or damaged.
- if you have viral or fungal infections in the ear, including infections caused by the varicella or herpes simplex virus.
- if you have otitis media (an infection or inflammation of the middle ear). Symptoms include ear pain, high temperature (fever), and a feeling of ear fullness.
Warnings and precautions
- use CIPROXINA only in the ears. Do not inject or ingest this medicine.
- if you experience any rash or notice the first signs of a rash on the skin or any other local allergic reaction, including hives, itching, or breathing problems, discontinue treatment immediately and consult your doctor.
- with the use of this medicine, you may become more susceptible to other infections. Consult your doctor if symptoms and signs persist after one week of treatment.
- the dropper cap contains latex, which may cause severe allergic reactions.
- contact your doctor if you experience blurred vision or other visual disturbances.
Children
CIPROXINA is not recommended for use in children under 2 years of age due to the lack of data in these patients.
Using CIPROXINA with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
It is recommended not to apply other medicines in the ear at the same time.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Driving and using machines
Treatment with CIPROXINAdoes not affect the ability to drive and use machines.
CIPROXINA contains benzyl alcohol
This medicine contains 90 mg of benzyl alcohol in each 10 ml vial, equivalent to 9 mg/ml. Benzyl alcohol may cause allergic reactions and moderate local irritation.
The dropper cap contains latex
The dropper cap contains latex. It may cause severe allergic reactions.
3. How to use CIPROXINA
This medicine should only be used in the ear. Do not inject or ingest this medicine.
Follow your doctor's instructions for administering CIPROXINA exactly. If in doubt, consult your doctor or pharmacist again.
Apply 3 drops into the ear canal of the affected ear, 2 times a day, in the morning and at night.
The usual treatment duration is 7 days. If symptoms persist after this, consult your doctor.
To ensure this medicine is effective, apply it regularly at the prescribed doses and for the duration indicated by your doctor.
The disappearance of symptoms does not mean you are completely cured. Any feeling of fatigue is not due to the treatment but to the infection. Reducing the dose or interrupting treatment will not affect this feeling and will only delay your recovery.
To correctly apply the drops, follow these steps:
- Wash your hands thoroughly.
- Remove the cap from the vial and place the dropper in the vial.
- Warm the vial immediately before use by holding it in the palm of your hand for a few minutes to avoid discomfort when the cold suspension comes into contact with your ears.
- Shake the vial before use.
- With your head tilted to the side opposite the affected ear, leaving it facing upwards, apply the drops into the affected ear.
- With your head still tilted, gently pull the earlobe forward and backward (Figure 1). This movement will allow the drops to penetrate the ear canal.
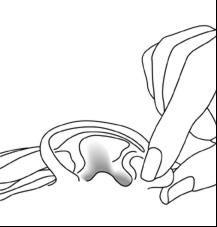 Figure 1
Figure 1
- Keep your head tilted to that side for about 5 minutes to facilitate the entry of the drops through the external ear canal. However, when you lift your head, some drops may spill out of the ear. You can wipe them away with a non-sterile absorbent paper.
- Repeat, if necessary, in the other ear.
It is very important to follow these instructions to achieve good efficacy of the medicine in the ear. When applying the drops to the ear, it is not recommended to keep your head vertical or move it too quickly, as some drops may be lost as they slide down your face and will not penetrate the ear canal.
Avoid the dropper coming into contact with the ears or fingers to limit the risk of contamination of the drops.
After completing the treatment, discard the remaining contents of the vial and do not store it for future use.
If you use more CIPROXINA than you should, do not apply more drops until the next dose.
In case of overdose or accidental ingestion, consult your doctor, pharmacist, or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use CIPROXINA, apply a single dose as soon as you remember, and continue with the next dose that was scheduled. However, if it is almost time for the next dose, do not apply the missed dose and continue with the next dose of your regular regimen. Do not apply a double dose to make up for missed doses.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, CIPROXINA can cause side effects, although not everybody gets them.
The following side effects have been observed with CIPROXINA:
Common
(may affect up to 1 in 10 people)
Ear effects: itching in the ear.
Uncommon
(may affect up to 1 in 100 people)
Ear effects: ear pain, ear congestion, ear discomfort, redness of the ear canal.
Other effects: dizziness, headache, nausea, skin peeling, fungal skin infection, hives, skin rash, itching, abnormal or decreased sensation in the skin, and presence of medication residue inside or around the ear.
Additional side effects have been reported for which the frequency is not known(cannot be estimated from the available data), including:
Ear effects: reduced hearing, ringing in the ears.
Eye effects: blurred vision
Other effects: allergy.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly to the Spanish Medicines Agency's Pharmacovigilance System: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of CIPROXINA
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the vial and carton after EXP. The expiry date is the last day of the month stated.
Do not store above 25°C.
Do not refrigerate or freeze.
Keep the container in the outer carton to protect it from light.
Keep the container tightly closed to protect it from moisture.
Once the vial is opened and the dropper is placed, the drops should be used within the next 14 days.
After completing the treatment, discard the remaining contents of the vial. Do not store it for future use.
Medicines should not be disposed of via wastewater or household waste. Place the empty packaging and any unused medicine in the pharmacy's SIGRE collection point. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and further information
Composition of CIPROXINA
- The active substances are ciprofloxacin 2 mg/ml and hydrocortisone 10 mg/ml.
- The other ingredients are polysorbate 20, sodium acetate, glacial acetic acid, benzyl alcohol, lecithin, sodium chloride, polyvinyl alcohol, and purified water.
Appearance of the product and contents of the pack
CIPROXINA is a white to off-white liquid (a suspension). It is presented in a carton containing a 10 ml glass vial with a screw cap made of polypropylene and, separately, a dropper (a polypropylene pipette, a polypropylene cap, and a latex cap).
Marketing authorisation holder
Novartis Farmacéutica, S.A.
Gran Via de les Corts Catalanes, 764
08013 - Barcelona, Spain
Manufacturer
Siegfried El Masnou, S.A
C/Camil Fabra, 58
08320 El Masnou – Barcelona
or
Novartis Farmacéutica S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona, Spain
or
Novartis Pharma GmbH
Roonstrasse 25
90429 Nuremberg, Germany
Date of last revision of this leaflet: September 2020
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS): http://www.aemps.gob.es
- Country of registration
- Average pharmacy price3.31 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CIPROXINA 2 mg/ml + 10 mg/ml OTIC SUSPENSION DROPSDosage form: OTIC SOLUTION, 0.25 mg / 3.49 mgActive substance: fluocinolone acetonide and antiinfectivesManufacturer: Zambon S.A.U.Prescription requiredDosage form: OTIC SOLUTION, 3 mg/ml + 0.25 mg/mlActive substance: fluocinolone acetonide and antiinfectivesManufacturer: Laboratorios Salvat S.A.Prescription requiredDosage form: OTIC SOLUTION, 3mg ciprofloxacin HCl; 0.25mg fluocinolone acetonideActive substance: fluocinolone acetonide and antiinfectivesManufacturer: Laboratorios Salvat S.A.Prescription required
Online doctors for CIPROXINA 2 mg/ml + 10 mg/ml OTIC SUSPENSION DROPS
Discuss questions about CIPROXINA 2 mg/ml + 10 mg/ml OTIC SUSPENSION DROPS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions








