CIMZIA 200 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN

How to use CIMZIA 200 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Cimzia 200 mg solution for injection in a pre-filled pen
certolizumab pegol
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Cimzia and what is it used for
- What you need to know before you use Cimzia
- How to use Cimzia
- Possible side effects
- Storing Cimzia
- Contents of the pack and other information
Your doctor will also give you a “Patient Alert Card” which contains important safety information that you need to know before you are given Cimzia and during treatment with this medicine. Keep this “Patient Alert Card” with you.
1. What is Cimzia and what is it used for
Cimzia contains the active substance certolizumab pegol, a fragment of a human antibody. Antibodies are proteins that recognize and bind specifically to other proteins. Cimzia binds to a specific protein called tumor necrosis factor alpha (TNFα). This TNFα is blocked by Cimzia, which reduces inflammation in diseases such as rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, and psoriasis. Medicines that bind to TNFα are also called TNF blockers.
Cimzia is used in adults to treat the following inflammatory diseases:
- rheumatoid arthritis,
- axial spondyloarthritis(including ankylosing spondylitis and axial spondyloarthritis without radiographic evidence of ankylosing spondylitis),
- psoriatic arthritis
- psoriasis
Rheumatoid Arthritis
Cimzia is used to treat rheumatoid arthritis. Rheumatoid arthritis is an inflammatory disease of the joints. If you have moderate to severe active rheumatoid arthritis, you may first be given other medicines, usually methotrexate. If you do not respond well to these medicines, you will be given Cimzia in combination with methotrexate to treat your rheumatoid arthritis. If your doctor decides that methotrexate is not suitable, Cimzia can be given alone.
Cimzia in combination with methotrexate can also be used to treat severe, progressive rheumatoid arthritis without prior use of methotrexate or other treatments.
Cimzia, when given in combination with methotrexate, is used to:
- reduce the signs and symptoms of your disease,
- slow down the damage to the cartilage and bones of the joints caused by the disease,
- improve your physical function and ability to perform daily activities.
Ankylosing Spondylitis and Axial Spondyloarthritis without radiographic evidence of Ankylosing Spondylitis
Cimzia is used to treat ankylosing spondylitis and axial spondyloarthritis without radiographic evidence of ankylosing spondylitis (sometimes referred to as non-radiographic axial spondyloarthritis). These diseases are inflammatory diseases of the spine. If you have ankylosing spondylitis or non-radiographic axial spondyloarthritis, you will first be treated with other medicines. If you do not respond well to these medicines, you will be given Cimzia to:
- reduce the signs and symptoms of your disease,
- improve your physical function and ability to perform daily activities.
Psoriatic Arthritis
Cimzia is used to treat active psoriatic arthritis. Psoriatic arthritis is an inflammatory disease of the joints, usually accompanied by psoriasis. If you have active psoriatic arthritis, you will first be given other medicines, usually methotrexate. If you do not respond well to these medicines, you will be given Cimzia in combination with methotrexate to:
- reduce the signs and symptoms of your disease,
- improve your physical function and ability to perform daily activities.
If your doctor decides that methotrexate is not a suitable treatment, Cimzia can be given alone.
Psoriasis
Cimzia is used to treat moderate to severe plaque psoriasis. Plaque psoriasis is an inflammatory disease of the skin that can also affect the scalp and nails.
Cimzia is used to reduce the inflammation of the skin and other signs and symptoms of the disease.
2. What you need to know before you use Cimzia
Do not use Cimzia
- If you are ALLERGIC(hypersensitive) to certolizumab pegol or any of the other ingredients of this medicine (listed in section 6).
- If you have a severe infection, including ACTIVE TUBERCULOSIS(TB).
- If you have MODERATE TO SEVERE HEART FAILURE. Tell your doctor if you have or have had any serious heart problems.
Warnings and precautions
Before starting treatment with Cimzia, tell your doctor if:
Allergic reactions
- If you experience ALLERGIC REACTIONSsuch as chest tightness, difficulty breathing, dizziness, swelling, or rash, stop using Cimzia and contact your doctor IMMEDIATELY. Some of these reactions may occur after the first administration of Cimzia.
- If you have ever had an allergic reaction to latex.
Infections
- If you have had RECURRENT INFECTIONSor OPPORTUNISTIC INFECTIONSor other diseases that increase the risk of infections (such as treatment with immunosuppressants, which are medicines that can reduce your ability to fight infections).
- If you have any infection or develop symptoms such as fever, wounds, fatigue, or dental problems. While you are being treated with Cimzia, you may be more likely to get an infection, including serious infections, or in rare cases, life-threatening infections.
- Cases of TUBERCULOSIS (TB)have been reported in patients treated with Cimzia. Your doctor will check you for signs or symptoms of tuberculosis before starting your treatment with Cimzia. This will include a thorough medical history, chest X-ray, and tuberculin test. The results of these tests should be recorded on your “Patient Alert Card”. If you are diagnosed with latent (inactive) tuberculosis, you may need to take medication to treat the tuberculosis before starting treatment with Cimzia. In rare cases, tuberculosis can develop during treatment, even if you have received preventive treatment for tuberculosis. It is very important that you tell your doctor if you have had tuberculosis or have been in contact with a patient with tuberculosis. If you develop symptoms of tuberculosis (persistent cough, weight loss, general feeling of being unwell, low-grade fever) or any other infection during or after treatment with Cimzia, contact your doctor immediately.
- If you are at risk of getting an infection with the HEPATITIS B VIRUS (HBV), if you are a carrier of HBV, or if you have an active HBV infection, Cimzia may increase the risk of HBV reactivation in people who carry this virus. If this happens, you must stop using Cimzia. Your doctor will do tests to check for HBV before starting treatment with Cimzia.
Heart failure
- If you have MILD HEART FAILUREand are being treated with Cimzia, your doctor will closely monitor your heart failure. It is important that you tell your doctor if you have or have had any serious heart problems. If you develop new symptoms of heart failure or if your existing symptoms get worse (e.g., difficulty breathing or swelling of the feet), you must contact your doctor immediately. Your doctor will decide if you should stop treatment with Cimzia.
Cancer
- It is rare, but cases of certain types of CANCERhave been observed in patients treated with Cimzia or other TNF blockers. People with more severe rheumatoid arthritis and who have had the disease for a long time may have a higher than average risk of developing lymphoma, a type of cancer that affects the lymphatic system. If you are being treated with Cimzia, you may have a higher risk of getting lymphoma or other types of cancer. There have also been rare cases of non-melanoma skin cancer in patients using Cimzia. Tell your doctor if you get any new skin lesions or if existing lesions change in appearance during or after treatment with Cimzia. There have been cases of cancer, including rare types, in children and adolescents taking TNF blockers, which in some cases were fatal (see below “Use in children and adolescents”).
Other diseases
- Patient with chronic obstructive pulmonary disease (COPD) or those who smoke heavily may have a higher risk of getting cancer with Cimzia treatment. If you have COPD or smoke heavily, you should discuss with your doctor whether treatment with a TNF blocker is suitable for you.
- If you have a disease of the nervous system, such as multiple sclerosis, your doctor will decide if you should use Cimzia.
- In some patients, the body may not be able to produce enough blood cells that help the body fight infections or those that help stop bleeding. If you have a persistent fever, bruising, or bleeding easily, or are very pale, you should contact your doctor immediately. Your doctor may decide to stop treatment with Cimzia.
- It is rare, but symptoms of a disease called lupus (e.g., persistent rash, fever, joint pain, and fatigue) may appear. Contact your doctor if you experience these symptoms. Your doctor may decide to stop treatment with Cimzia.
Vaccinations
- Tell your doctor if you have been given or are going to be given a vaccine. Some vaccines (live) should not be given while you are being treated with Cimzia.
- Some vaccines may cause infections. If you have been treated with Cimzia during pregnancy, your baby may have a higher risk of getting these infections until approximately five months after your last dose of Cimzia. It is important that you tell your baby’s doctors and other healthcare professionals about your treatment with Cimzia so that they can decide when your baby should be vaccinated.
Surgical or dental procedures
- Tell your doctor if you are going to have a surgical or dental procedure. Tell the surgeon or dentist that you are being treated with Cimzia and show them your “Patient Alert Card”.
Children and adolescents
The use of Cimzia is not recommended in patients under 18 years of age.
Using Cimzia with other medicines
DO NOTuse Cimzia if you are using the following medicines to treat rheumatoid arthritis:
- anakinra
- abatacept
If you are unsure, ask your doctor.
Cimzia can be used with:
- methotrexate,
- corticosteroids or
- painkillers, including non-steroidal anti-inflammatory drugs (also called NSAIDs).
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Cimzia should only be used during pregnancy if clearly necessary. If you are a woman of childbearing age, discuss with your doctor the use of adequate contraception while using Cimzia. For women who intend to become pregnant, contraception may be considered for 5 months after the last administration of Cimzia.
If you have been treated with Cimzia during pregnancy, your baby may have a higher risk of getting an infection. It is important that you tell your baby’s doctors and other healthcare professionals about your treatment with Cimzia before your baby is vaccinated (for more information, see the section on vaccinations).
Cimzia can be used during breastfeeding.
Driving and using machines
Cimzia has a minor influence on the ability to drive and use machines. Dizziness (including a feeling that the room is spinning, blurred vision, and fatigue) may occur after using Cimzia.
Cimzia containssodium acetate and sodium chloride
This medicine contains less than 23 mg (1 mmol) of sodium per 400 mg, so it is essentially “sodium-free”.
3. How to use Cimzia
Follow the instructions for administration of this medicine exactly as told by your doctor or pharmacist. If you are unsure, ask your doctor or pharmacist again.
Rheumatoid Arthritis
- The starting dose for adults with rheumatoid arthritis is 400 mg given at weeks 0, 2, and 4.
- This dose is followed by a maintenance dose of 200 mg every 2 weeks. If you respond to the medicine, your doctor may prescribe an alternative maintenance dose of 400 mg every 4 weeks.
- Methotrexate treatment is continued during use of Cimzia. If your doctor decides that methotrexate is not suitable, Cimzia can be given alone.
Axial Spondyloarthritis
- The starting dose for adults with axial spondyloarthritis is 400 mg given at weeks 0, 2, and 4.
- This dose is followed by a maintenance dose of 200 mg every 2 weeks (from week 6) or 400 mg every 4 weeks (from week 8), as directed by your doctor. If you have been treated with Cimzia for at least 1 year and respond to the medicine, your doctor may prescribe a reduced maintenance dose of 200 mg every 4 weeks.
Psoriatic Arthritis
- The starting dose for adults with psoriatic arthritis is 400 mg given at weeks 0, 2, and 4.
- This dose is followed by a maintenance dose of 200 mg every 2 weeks. If you respond to the medicine, your doctor may prescribe an alternative maintenance dose of 400 mg every 4 weeks.
- Methotrexate treatment is continued during use of Cimzia. If your doctor decides that methotrexate is not suitable, Cimzia can be given alone.
Psoriasis
- The initial dose for adults with psoriasis is 400 mg every 2 weeks given at weeks 0, 2, and 4.
- This is followed by a maintenance dose of 200 mg every 2 weeks or 400 mg every 2 weeks, as directed by your doctor.
How to use Cimzia
Cimzia will normally be given to you by a specialist doctor or healthcare professional. Cimzia will be given as an injection (200 mg dose) or 2 injections (400 mg dose) under the skin (subcutaneously, abbreviated as SC). It is usually injected into the thigh or stomach. However, do not inject into areas where the skin is red, bruised, or hard.
Instructions for self-injecting Cimzia
After proper training, your doctor may also allow you to self-inject Cimzia. Read the instructions on how to inject Cimzia at the end of this leaflet.
If your doctor has decided that you can inject this medicine, you must follow up with them before continuing to self-inject:
- after 12 weeks if you have rheumatoid arthritis, axial spondyloarthritis, or psoriatic arthritis; or
- after 16 weeks if you have psoriasis.
This is so that your doctor can determine if Cimzia is working for you or if another treatment should be considered.
If you use more Cimzia than you should
If your doctor has decided that you can inject this medicine and you accidentally inject Cimzia more frequently than prescribed, you must tell your doctor. Always carry your “Patient Alert Card” and the medicine packaging, even if it is empty.
If you forget to use Cimzia
If your doctor has decided that you can inject this medicine and you miss an injection, you should inject the next dose of Cimzia as soon as you remember. Then, inject the next doses as you were told. Talk to your doctor afterwards and inject the next doses following the instructions given by your doctor.
If you stop treatment with Cimzia
Do not stop treatment with Cimzia without talking to your doctor first.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Report IMMEDIATELYto your doctor if you notice any of the following adverse effects:
- severe rash, hives or other signs of allergic reaction (urticaria)
- swollen face, hands, feet (angioedema)
- breathing or swallowing problems (multiple causes for these symptoms)
- difficulty breathing (dyspnea) with exertion or when lying down, or swelling of the feet (heart failure)
- symptoms of blood disorders such as persistent fever, bruising, bleeding, pallor (pancytopenia, anemia, low platelet count, low white blood cell count)
- severe skin eruptions. These can appear as red, circular patches or spots, often with central blisters on the trunk, skin peeling, ulcers in the mouth, throat, nose, genitals, and eyes, and may be preceded by fever and flu-like symptoms (Stevens-Johnson syndrome).
Report to your doctor AS SOON AS POSSIBLEif you notice any of the following adverse effects:
- signs of infection such as fever, malaise, wounds, dental problems, burning when urinating
- feeling of weakness or fatigue
- cough
- tingling
- numbness
- double vision
- weakness in arms or legs
- bruises or sores that do not heal
The symptoms described above may be due to some of the adverse effects listed below, which have been observed with Cimzia:
Frequent (may affect up to 1 in 10 patients):
- bacterial infections in any location (pus accumulation)
- viral infections (including herpes labialis, herpes zoster, and flu)
- fever
- high blood pressure
- rash or itching
- headaches (including migraine)
- sensory disturbances such as numbness, tingling, burning sensation
- feeling of weakness and general malaise
- pain
- blood disorders
- liver problems
- injection site reactions
- nausea
Uncommon (may affect up to 1 in 100 patients):
- allergic diseases including rhinitis and allergic reactions to the medicine (including anaphylactic shock)
- antibodies directed against normal tissue
- lymphatic and blood system cancer such as lymphoma and leukemia
- solid tumors
- skin cancer, precancerous skin lesions
- benign tumors (non-cancerous) and cysts (including skin cysts)
- heart problems including weakened heart muscle, heart failure, myocardial infarction, chest discomfort or pressure, abnormal heart rhythm including irregular heartbeats
- edema (swelling in the face or legs)
- lupus symptoms (immune system disease that affects connective tissue): joint pain, skin rash, photosensitivity, and fever
- inflammation of blood vessels
- sepsis (severe infection that can lead to organ failure, shock, or death)
- tuberculous infection
- fungal infections (occur when the ability to fight infections is reduced)
- respiratory disorders and inflammation (including asthma, shortness of breath, cough, sinus blockage, pleuritis, or difficulty breathing)
- stomach problems including abdominal fluid accumulation, ulcers (including oral ulcers), perforation, distension, inflammation, heartburn, stomach discomfort, dry mouth
- biliary problems
- muscle problems including increased muscle enzymes
- changes in blood salt levels
- changes in blood cholesterol and lipid levels
- blood clots in veins or lungs
- bleeding or bruising
- changes in blood cell count, including low red blood cell count (anemia), low platelet count, increased platelet count
- swollen lymph nodes
- flu-like symptoms, chills, altered temperature perception, night sweats, flushing
- anxiety and mood disorders such as depression, appetite disorders, weight changes
- vertigo (dizziness)
- ringing in the ears
- fainting, including loss of consciousness
- disorders of the nerves in the extremities including symptoms of numbness, tingling, burning sensation, dizziness, tremors
- skin disorders such as new episode or worsening of psoriasis, skin inflammation (such as eczema), sweat gland disorders, ulcers, photosensitivity, acne, hair loss, skin color changes, nail separation, dry skin, and wounds
- wound healing problems
- urinary and kidney problems including altered renal function, blood in urine, and urinary disorders
- menstrual cycle disorders (monthly period) including lack of bleeding or excessive bleeding, or irregular bleeding
- breast disorders
- eye and eyelid inflammation, vision changes, tear problems
- increase in some blood parameters (increase in blood alkaline phosphatase)
- prolonged coagulation times
Rare (may affect up to 1 in 1,000 patients):
- gastrointestinal cancer, melanoma
- lung inflammation (interstitial lung disease, lung inflammation)
- stroke, blockage of blood vessels (arteriosclerosis), poor blood circulation that causes numbness and paleness of the fingers and toes (Raynaud's phenomenon), purple spots, skin discoloration, small veins near the skin surface may become visible
- pericarditis
- cardiac arrhythmia
- spleen enlargement
- increase in red blood cell mass
- abnormal white blood cell morphology
- gallstone formation
- kidney problems (including nephritis)
- immune system disorders such as sarcoidosis (rash, joint pain, fever), serum sickness, fat tissue inflammation, angioneurotic edema (swelling of lips, face, throat)
- thyroid disorders (goiter, fatigue, weight loss)
- increase in body iron levels
- increase in blood uric acid levels
- suicide attempt, mental disorder, delirium
- inflammation of the auditory nerve, vision, or face, altered coordination or balance
- increased gastrointestinal motility
- fistula (channel that connects an organ to another) (in any area)
- mouth alterations including pain when swallowing
- skin peeling, vesicular disorders, hair texture changes
- sexual dysfunction
- crisis
- worsening of a disease called dermatomyositis (which appears as muscle weakness accompanied by a skin rash)
- Stevens-Johnson syndrome (a severe skin disease in which the first symptoms include general malaise, fever, headache, and rash)
- inflammatory skin eruption (erythema multiforme)
- lichenoid reactions (pruritic reddish-purple rash and/or thick grayish-white lines on the mucous membranes)
Unknown (frequency cannot be estimated from available data):
- multiple sclerosis*
- Guillain-Barré syndrome*
- Merkel cell carcinoma (a type of skin cancer)*
- Kaposi's sarcoma (a rare cancer related to human herpesvirus 8 infection. Kaposi's sarcoma usually manifests more frequently as purple skin lesions).
*These events have been associated with this class of medicines, but the incidence in Cimzia is unknown.
Other Adverse Effects
When Cimzia has been used to treat other diseases, the following uncommon adverse effects have occurred:
- gastrointestinal stenosis (narrowing of part of the digestive system).
- gastrointestinal obstructions (blockage of the digestive system).
- general physical health deterioration.
- spontaneous abortion.
- azoospermia (lack of sperm production).
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is an adverse effect that does not appear in this leaflet. You can also report them directly through the national reporting system included in Appendix V.
By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Cimzia
Keep this medicine out of sight and reach of children.
Do not use this medicine after the expiration date that appears on the packaging after CAD and on the pen after EXP. The expiration date is the last day of the month indicated.
Store in a refrigerator (2 °C-8 °C).
Do not freeze.
Store the pre-filled pen in the outer packaging to protect it from light.
The pre-filled pens can be stored at room temperature (not above 25 °C) for a single maximum period of 10 days, protected from light. At the end of this period, the pre-filled pens must be used or discarded.
Do not use this medicine if the solution is discolored, cloudy, or if you can see particles in it.
Medicines should not be thrown away through wastewater or household waste. Ask your pharmacist how to dispose of the packaging and medicines that are no longer needed. This will help protect the environment.
6. Container Contents and Additional Information
Cimzia Composition
- The active ingredient is certolizumab pegol. Each pre-filled pen contains 200 mg of certolizumab pegol in 1 ml.
- The other components are: sodium acetate, sodium chloride, and water for injectable preparations (see "Cimzia contains sodium acetate and sodium chloride" in section 2).
Product Appearance and Container Contents
Cimzia is supplied as a ready-to-use injectable solution in a pre-filled pen (AutoClicks). The solution is clear to opalescent, colorless to yellowish.
A container of Cimzia contains:
- two pre-filled AutoClicks pens containing the solution and
- two alcohol swabs (for cleaning the selected injection areas).
Containers with 2 pre-filled pens and 2 alcohol swabs, a multiple container containing 6 pre-filled pens (3 containers of 2) and 6 alcohol swabs (3 containers of 2), and a multiple container containing 10 pre-filled pens (5 containers of 2) and 10 alcohol swabs (5 containers of 2) are available.
Only some package sizes may be marketed.
Marketing Authorization Holder
UCB Pharma SA
Allée de la Recherche 60
B-1070 Brussels
Belgium
Manufacturer
UCB Pharma S.A.
Chemin du Foriest
B-1420 Braine l'Alleud
Belgium
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien UCB Pharma S.A./NV Tel/Tél: + 32 / (0)2 559 92 00 | Lietuva UCB Pharma Oy Finland Tel: + 358 9 2514 4221 (Suomija) |
| Luxembourg/Luxemburg UCB Pharma S.A.//NV Tél/Tel: + 32 / (0)2 559 92 00 (Belgique/Belgien) |
Ceská republika UCB s.r.o. Tel: + 420 221 773 411 | Magyarország UCB Magyarország Kft. Tel.: + 36-(1) 391 0060 |
Denmark UCB Nordic A/S Tlf.: + 45 / 32 46 24 00 | Malta Pharmasud Ltd. Tel: + 356 / 21 37 64 36 |
Deutschland UCB Pharma GmbH Tel: + 49 / (0)2173 48 4848 | Nederland UCB Pharma B.V. Tel.: + 31 / (0)76-573 11 40 |
Eesti UCB Pharma Oy Finland Tel: + 358 9 2514 4221 (Soome) | Norge UCB Nordic A/S Tlf: + 47 / 67 16 5880 |
Ελλ?δα UCB Α.Ε. Τηλ: + 30 / 2109974000 | Österreich UCB Pharma GmbH Tel: + 43-(0)1 291 80 00 |
España UCB Pharma S.A. Tel: + 34 / 91 570 34 44 | Polska UCB Pharma Sp. z o.o. / VEDIM Sp. z o.o. Tel.: + 48 22 696 99 20 |
France UCB Pharma S.A. Tél: + 33 / (0)1 47 29 44 35 | Portugal UCB Pharma (Produtos Farmacêuticos), Lda Tel: + 351 / 21 302 5300 |
Hrvatska Medis Adria d.o.o. Tel: +385 (0) 1 230 34 46 | România UCB Pharma Romania S.R.L. Tel: + 40 21 300 29 04 |
Ireland UCB (Pharma) Ireland Ltd. Tel: + 353 / (0)1-46 37 395 | Slovenija Medis, d.o.o. Tel: + 386 1 589 69 00 |
Ísland Vistor hf. Tel: + 354 535 7000 | Slovenská republika UCB s.r.o., organizačná zložka Tel: + 421 (0) 2 5920 2020 |
Italia UCB Pharma S.p.A. Tel: + 39 / 02 300 791 | Suomi/Finland UCB Pharma Oy Finland Puh/Tel: + 358 9 2514 4221 |
Κ?προς Lifepharma (Z.A.M.) Ltd Τηλ: + 357 22 056300 | Sverige UCB Nordic A/S Tel: + 46 / (0) 40 29 49 00 |
Latvija UCB Pharma Oy Finland Tel: + 358 9 2514 4221 |
Date of Last Revision of this Leaflet:{MM/YYYY}.
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu/.
<-------------------------------------------------------------------------------------------------------------------------
INSTRUCTIONS FOR INJECTING CIMZIA WITH A PRE-FILLED PEN
After proper training, you may inject yourself or have another person, such as a family member or friend, inject Cimzia. The following instructions explain how to use the pre-filled pen (AutoClicks) to inject Cimzia. Read them carefully and follow them step by step. Your doctor or other healthcare professional will teach you how to administer the injection yourself. Do not attempt to inject yourself before you are sure you understand how to prepare and administer the injection.
Below is a diagram of a pre-filled AutoClicks pen.
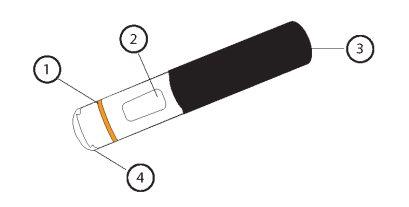
1: Orange band
2: Viewfinder
3: Black handle
4: Transparent cap
- Preparation
- Remove the Cimzia container from the refrigerator.
- If the seal(s) is broken or missing – do not use and contact your pharmacist.
- Remove the following items from the Cimzia box and place them on a flat and clean surface:
- - One or two pre-filled AutoClicks pens, depending on your prescribed dose
- - One or two alcohol swabs
- Check the expiration date of the pre-filled pen and the box. Do not use Cimzia after the expiration date stated on the box after CAD and on the pre-filled pen after EXP. The expiration date is the last day of the month indicated.
- Allow the pre-filled AutoClicks pen to reach room temperature. This will take 30 to 45 minutes. This will help reduce discomfort during injection.
- Do not heat the pre-filled pen - let it warm up on its own.
- Do not remove the cap until you are ready to inject.
- Wash your hands thoroughly.
- Selection and Preparation of the Injection Site
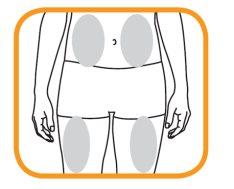
- Choose an area on your thigh or stomach.
|
- Each new injection should be administered in a separate area from the previous injection site.
- - Never inject into an area where the skin is red, bruised, or hardened.
- - Clean the injection site with the included alcohol swab, following a circular motion from inside out.
- - Do not touch this area again before the injection.
- Injection
- The pre-filled AutoClicks pen is designed to function precisely and safely. However, if any of the following steps go wrong and/or if you feel unsure about the injection process, contact your doctor or pharmacist.
- Do not shake the pre-filled pen.
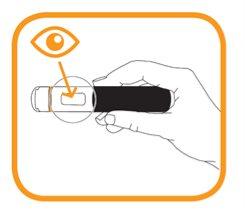
Check the medication through the viewfinder.
- Do not use this medication if the solution is discolored, cloudy, or if you can see particles in it.
- You may observe air bubbles - this is normal. Injecting a subcutaneous solution that contains air bubbles is harmless.
- Hold the pre-filled pen firmly with one hand around the black handle.
- Take the transparent cap with the other hand and pull it straight off. Do not twist the cap when removing it, as this could jam the internal mechanism.
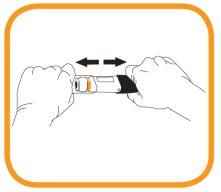
- Inject within 5 minutes after removing the cap. Do not replace the cap.
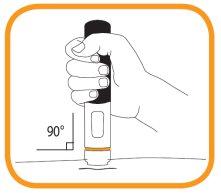
- Although the needle is hidden from view, it is now exposed. Do not attempt to touch the needle, as this could activate the pre-filled pen. Hold the pre-filled pen directly against the skin (at a 90-degree angle) that you previously cleaned ("injection site").
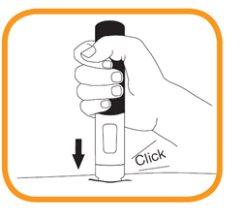
- Press the pre-filled pen firmly against the skin. The injection starts when you hear the first "click" and the orange band disappears from the bottom of the pre-filled pen.
- Continue to hold the pre-filled pen firmly in place against the skin until you hear a second "click" and the viewfinder turns orange. This may take 15 seconds. At this point, the injection will be complete. If the viewfinder turns orange and you hear the second click, this means the injection is complete. If you feel unsure about the injection process, contact your doctor or pharmacist. Do not attempt to repeat the injection process without speaking to your doctor or pharmacist.
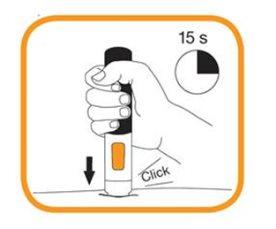
- The needle will move back automatically into the empty pen. Do not attempt to touch the needle.
- You can now remove the pen by pulling it straight and carefully away from the skin.
- Using a little cotton, press on the injection site for a few seconds.
- Do not rub the injection site.
- If necessary, you can cover the injection site with a plaster.
- After Use
- Do not reuse the pre-filled pen. You do not need to replace the cap.
- After the injection, immediately discard the used pen(s) in a special container as instructed by your doctor, nurse, or pharmacist.
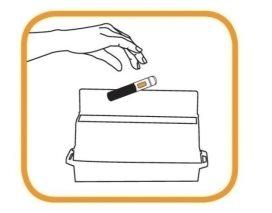
- Keep the container out of sight and reach of children.
- If you need to administer a second injection as prescribed by your doctor, repeat the injection process starting from step 2.
.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CIMZIA 200 mg SOLUTION FOR INJECTION IN A PRE-FILLED PENDosage form: INJECTABLE, 200 mg/mlActive substance: certolizumab pegolManufacturer: Ucb PharmaPrescription requiredDosage form: INJECTABLE, see Pharmaceutical Details IIActive substance: certolizumab pegolManufacturer: Ucb PharmaPrescription requiredDosage form: INJECTABLE, 20 mgActive substance: adalimumabManufacturer: Amgen Europe B.V.Prescription required
Online doctors for CIMZIA 200 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN
Discuss questions about CIMZIA 200 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions