BYOOVIZ 10 mg/ml INJECTABLE SOLUTION

How to use BYOOVIZ 10 mg/ml INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Adult Patient
Byooviz 10 mg/ml Solution for Injection
ranibizumab
This medicinal product is subject to additional monitoring, which will allow for the quick identification of new safety information. You can help by reporting any side effects you may experience. The last section of section 4 will tell you how to report side effects.
ADULTS
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Byooviz and what is it used for
- What you need to know before you are given Byooviz
- How Byooviz is given
- Possible side effects
- Storage of Byooviz
- Contents of the pack and other information
1. What is Byooviz and what is it used for
What is Byooviz
Byooviz is a solution that is injected into the eye. Byooviz belongs to a group of medicines called anti-angiogenic agents. It contains the active substance called ranibizumab.
What is Byooviz used for
Byooviz is used in adults to treat several eye diseases that cause vision problems.
These diseases are the result of damage to the retina (the light-sensitive layer at the back of the eye) caused by:
- The growth of abnormal blood vessels that leak fluid. This is seen in diseases such as age-related macular degeneration (AMD) and proliferative diabetic retinopathy (PDR, a disease caused by diabetes). It may also be associated with choroidal neovascularization (CNV) due to pathologic myopia (PM), angioid streaks, central serous chorioretinopathy, or inflammatory CNV.
- Macular edema (swelling of the center of the retina). The cause of this swelling may be diabetes (a disease known as diabetic macular edema [DME]) or a blockage of the retinal veins in the retina (a disease known as retinal vein occlusion [RVO]).
How Byooviz works
Byooviz recognizes and binds specifically to a protein called vascular endothelial growth factor A (VEGF-A) present in the eyes. In excess, VEGF-A causes the growth of abnormal blood vessels and swelling in the eye that can lead to vision problems in diseases such as AMD, DME, PDR, RVO, PM, and CNV. By binding to VEGF-A, Byooviz can prevent it from working and prevent such abnormal growth and swelling.
In these diseases, Byooviz can help stabilize and, in many cases, improve your vision.
2. What you need to know before you are given Byooviz
You must not be given Byooviz
- If you are allergic to ranibizumab or any of the other ingredients of this medicine (listed in section 6).
- If you have an infection in the eye or around the eye.
- If you have pain or redness (severe intraocular inflammation) in the eye.
Warnings and precautions
Talk to your doctor before you are given Byooviz.
- Byooviz is given by injection into the eye. Occasionally, after treatment with Byooviz, an infection in the inner eye, pain or redness (inflammation), detachment or tear of one of the layers in the back of the eye (retinal detachment and retinal pigment epithelial tear), or clouding of the lens (cataract) may occur. It is essential to identify and treat such an infection or retinal detachment as soon as possible. Tell your doctor immediately if you notice signs such as eye pain or increased discomfort in the eye, worsening redness in the eye, blurred vision or decreased vision, an increase in the number of small spots in your vision, or increased sensitivity to light.
- In some patients, shortly after the injection, the pressure in the eye may increase for a short period. You may not be aware of this, so your doctor may monitor your eye pressure after each injection.
- Tell your doctor if you have had eye diseases or have received any treatment in the eyes before, or if you have had a stroke or have had transient signs of a stroke (weakness or paralysis of a limb or face, difficulty speaking or understanding). This information will be taken into account to assess whether Byooviz is the right treatment for you.
For more detailed information on the side effects that may occur during treatment with Byooviz, see section 4 ("Possible side effects").
Children and adolescents (under 18 years)
The use of Byooviz in children and adolescents has not been established and is therefore not recommended.
Other medicines and Byooviz
Tell your doctor if you are using, have recently used, or might use any other medicines.
Pregnancy and breastfeeding
- Women who may become pregnant should use an effective method of contraception during treatment and for at least three months after the last injection of Byooviz.
- There is no experience with the use of Byooviz in pregnant women. Byooviz should not be used during pregnancy unless the potential benefit outweighs the potential risk to the fetus. If you are pregnant, think you may be pregnant, or plan to become pregnant, consult your doctor before treatment with Byooviz.
- Small amounts of ranibizumab may pass into breast milk, so the use of Byooviz is not recommended during breastfeeding. Consult your doctor or pharmacist before treatment with Byooviz.
Driving and using machines
After treatment with Byooviz, you may experience temporary blurred vision. If this happens, do not drive or use machines until this symptom disappears.
3. How Byooviz is given
Byooviz is given by an ophthalmologist as a single injection into the eye under local anesthesia. The usual dose of an injection is 0.05 ml (which contains 0.5 mg of active substance). The interval between two doses given in the same eye should be at least four weeks. All injections will be given by an ophthalmologist.
To prevent infection, before the injection, your doctor will carefully clean your eye. Your doctor will also give you a local anesthetic to reduce or prevent any pain you may feel with the injection.
Treatment is started with an injection of Byooviz every month. Your doctor will monitor the disease in your eye and, depending on how you respond to treatment, will decide whether you need further treatment and when you need to be treated.
At the end of the leaflet, in the section "How to prepare and administer Byooviz", detailed instructions for use are given.
Elderly patients (65 years and over)
Byooviz can be used in people 65 years of age or older, and no dose adjustment is necessary.
Before stopping treatment with Byooviz
If you are considering stopping treatment with Byooviz, go to your next appointment and discuss it with your doctor beforehand. Your doctor will advise you and decide how long you should be treated with Byooviz.
If you have any further questions on the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The side effects associated with the use of Byooviz are either due to the medicine itself or the injection procedure, and most affect the eye.
The following are the most serious side effects:
Common serious side effects(may affect up to 1 in 10 patients): detachment or tear of the layer in the back of the eye (retinal detachment and retinal pigment epithelial tear), resulting in flashes of light with floating particles that progress to transient vision loss or clouding of the lens (cataract).
Uncommon serious side effects(may affect up to 1 in 100 patients): blindness, infection of the eyeball (endophthalmitis) with inflammation of the inner eye.
The symptoms you may experience are eye pain or increased discomfort in the eye, worsening redness in the eye, blurred vision or decreased vision, an increase in the number of small spots in your vision, or increased sensitivity to light. Tell your doctor immediately if you experience any of these side effects.
The following are the most commonly reported side effects:
Very common side effects(may affect more than 1 in 10 patients)
Eye side effects include: eye inflammation, bleeding in the back of the eye (retinal hemorrhage), visual disturbances, eye pain, small particles or spots in the vision (floaters), blood in the eye, eye irritation, feeling of having something in the eye, increased tear production, inflammation or infection of the eyelid margin, dry eye, redness or itching of the eye, and increased pressure in the eye.
Non-eye side effects include: sore throat, nasal congestion, runny nose, headache, and joint pain.
The following are other side effects that may occur after treatment with Byooviz:
Common side effects
Eye side effects include: decreased sharpness of vision, swelling of a section of the eye (uvea, cornea), inflammation of the cornea (front part of the eye), small marks on the surface of the eye, blurred vision, bleeding at the injection site, bleeding in the eye, eye discharge with itching, redness, and swelling (conjunctivitis), sensitivity to light, eye discomfort, eyelid swelling, eyelid pain.
Non-eye side effects include: urinary tract infection, low red blood cell count (with symptoms such as fatigue, difficulty breathing, dizziness, paleness), anxiety, cough, nausea, allergic reactions such as rash, hives, itching, and redness of the skin.
Uncommon side effects
Eye side effects include: inflammation and bleeding in the front part of the eye, accumulation of pus in the eye, changes in the central part of the eye surface, pain or irritation at the injection site, abnormal sensation in the eye, eyelid irritation.
Reporting of side effects
If you experience any side effects, talk to your doctor, even if they are not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Byooviz
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the carton after CAD and on the label of the vial after EXP. The expiry date is the last day of the month shown.
- Store in a refrigerator (between 2°C and 8°C). Do not freeze.
- Before use, the unopened vial can be stored at temperatures not above 30°C for a maximum of 2 months.
- Store the vial in the outer packaging to protect it from light.
- Do not use any packaging that is damaged.
6. Container Contents and Additional Information
Byooviz Composition
- The active substance is ranibizumab. Each ml contains 10 mg of ranibizumab. Each vial contains 2.3 mg of ranibizumab in 0.23 ml of solution. This provides a sufficient amount to deliver a single dose of 0.05 ml, which contains 0.5 mg of ranibizumab.
- The other components are a,a-trehalose dihydrate; histidine hydrochloride monohydrate; histidine; polysorbate 20; water for injectable preparations.
Product Appearance and Container Contents
Byooviz is an injectable solution contained in a vial (0.23 ml). The solution is clear, colorless to pale yellow, and aqueous.
There are two types of packaging available:
Single Vial Packaging
Packaging containing a glass vial with ranibizumab, with a chlorobutyl rubber stopper. The vial is for single use.
Packaging with Vial + Filter Needle + Injection Needle
Packaging containing a glass vial with ranibizumab, with a chlorobutyl rubber stopper, a blunt filter needle (18G x 1½″, 1.2 mm x 40 mm, 5 microns) for extracting the vial contents, and an injection needle (30G x ½″, 0.3 mm x 13 mm). All components are for single use.
Only some packaging types may be marketed.
Marketing Authorization Holder and Manufacturer
Samsung Bioepis NL B.V.
Olof Palmestraat 10
2616 LR Delft
Netherlands
Date of Last Revision of this Leaflet: July 2025
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu
THIS INFORMATION IS INTENDED ONLY FOR HEALTHCARE PROFESSIONALS:
See also section 3 "How to Administer Byooviz".
How to Prepare and Administer Byooviz in Adults
Single-use vial. For intravitreal use only.
Byooviz should be administered by an ophthalmologist with experience in administering intravitreal injections.
In exudative age-related macular degeneration (AMD), neovascular (wet) age-related macular degeneration (NVC), retinal vein occlusion (RVO), and visual impairment due to diabetic macular edema (DME) or edema secondary to branch retinal vein occlusion (BRVO), the recommended dose of Byooviz is 0.5 mg administered as a single intravitreal injection. This corresponds to an injection volume of 0.05 ml. The interval between two doses injected into the same eye should be at least four weeks.
Treatment is initiated with one injection per month until maximum visual acuity is achieved and/or there are no signs of disease activity, i.e., no change in visual acuity or other signs and symptoms of the disease under continued treatment. In patients with exudative AMD, DME, RVO, and BRVO, three or more consecutive monthly injections may initially be necessary.
From that point on, monitoring and treatment intervals should be determined based on medical judgment and the disease activity, as assessed by visual acuity and/or anatomical parameters.
Treatment with Byooviz should be discontinued if, in the physician's judgment, visual and anatomical parameters indicate that the patient is not benefiting from continued treatment.
Monitoring to determine disease activity may include clinical examination, functional testing, or imaging techniques (e.g., optical coherence tomography or fluorescein angiography).
If patients are being treated according to a treat-and-extend regimen, once maximum visual acuity is achieved and/or there are no signs of disease activity, treatment intervals can be gradually extended until signs of disease activity or visual impairment recur. In the case of exudative AMD, the treatment interval should not be extended by more than two weeks at a time, and in the case of DME, it can be extended up to one month at a time. For RVO and BRVO, treatment intervals can also be gradually extended; however, available data are insufficient to determine the duration of these intervals. If disease activity recurs, the treatment interval should be shortened accordingly.
Treatment of visual impairment due to NVC should be determined individually for each patient based on disease activity. Some patients may require only one injection during the first 12 months; others may require more frequent treatment, including monthly injections. In the case of NVC secondary to pathologic myopia (PM), many patients may require only one or two injections during the first year.
Ranibizumab and Laser Photocoagulation in DME and Edema Secondary to Branch Retinal Vein Occlusion (BRVO)
There is some experience with ranibizumab administered concomitantly with laser photocoagulation. When administered on the same day, ranibizumab should be administered at least 30 minutes after laser photocoagulation. Ranibizumab may be administered in patients who have received prior laser photocoagulation.
Ranibizumab and Photodynamic Therapy with Verteporfin in NVC Secondary to Pathologic Myopia (PM)
There is no experience with the concomitant administration of ranibizumab and verteporfin.
Before administering Byooviz, the absence of particles and discoloration should be visually checked.
The injection procedure should be carried out under aseptic conditions, including surgical hand washing, use of sterile gloves, a sterile field, a sterile blepharostat (or equivalent), and the availability of a sterile paracentesis needle (if necessary). Before performing the intravitreal injection procedure, the patient's medical history should be thoroughly evaluated for hypersensitivity reactions. Before injection, adequate anesthesia and a broad-spectrum topical microbicide should be administered to disinfect the periocular skin, eyelid, and ocular surface, according to local practice.
Single Vial Packaging
The vial is for single use. After injection, any unused product should be discarded. No vial showing signs of damage or tampering should be used. Sterility can only be guaranteed if the container seal remains intact.
For preparation and intravitreal injection, the following medical devices (for single use) are required:
- a 5 µm filter needle (18G)
- a 30G x ½″ injection needle
- a 1 ml sterile syringe (including a 0.05 ml mark)
These medical devices are not included in the Byooviz packaging.
Packaging with Vial + Filter Needle + Injection Needle
All components are sterile and for single use. No component with packaging showing signs of damage or tampering should be used. Sterility can only be guaranteed if the component packaging seal remains intact. Reuse may lead to infection or other disease/injury.
For preparation and intravitreal injection, the following medical devices (for single use) are required:
- a 5 µm filter needle (18G x 1½″, 1.2 mm x 40 mm, supplied)
- a 30G x ½″ injection needle (0.3 mm x 13 mm, supplied)
- a 1 ml sterile syringe (including a 0.05 ml mark, not included in the Byooviz packaging)
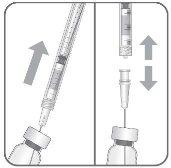
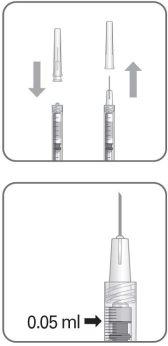
To prepare Byooviz for intravitreal administration in adult patients, follow these instructions:
|
|
|
|
|
Note: Hold the injection needle by the hub while removing the cap.
Note: Do not wipe the injection needle. Do not pull the plunger back. |
The injection needle should be inserted 3.5-4.0 mm behind the limbus into the vitreous cavity, avoiding the horizontal meridian and aiming toward the center of the globe. Then, the 0.05 ml injection volume should be released; subsequent injections should be applied to a different scleral location each time.
After injection, do not cover the needle with the cap or separate it from the syringe. Discard the used syringe along with the needle in a puncture-resistant container or dispose of according to local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BYOOVIZ 10 mg/ml INJECTABLE SOLUTIONDosage form: INJECTABLE, 10 mg/mLActive substance: ranibizumabManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: INJECTABLE, 10 mg/mLActive substance: ranibizumabManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: INJECTABLE, 10 mg/mlActive substance: ranibizumabManufacturer: Midas Pharma GmbhPrescription required
Online doctors for BYOOVIZ 10 mg/ml INJECTABLE SOLUTION
Discuss questions about BYOOVIZ 10 mg/ml INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions