
BILAXTEN 6 mg/ml EYE DROPS SOLUTION

How to use BILAXTEN 6 mg/ml EYE DROPS SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Bilaxten 6 mg/ml Eye Drops Solution
bilastine
Read this package leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Bilaxten and what is it used for
- What you need to know before you use Bilaxten
- How to use Bilaxten
- Possible side effects
- Storage of Bilaxten
- Contents of the pack and other information
1. What is Bilaxten and what is it used for
This medicine contains bilastine, which belongs to a group of medicines called antihistamines. Antihistamines work by preventing the effects of a substance called histamine, which the body produces as part of an allergic reaction.
This medicine is used to treat the signs and symptoms of eye disorders that occur with seasonal allergic conjunctivitisin adults and children from 2 years of age.
This medicine is also used to treat the signs and symptoms of eye disorders caused by an allergy to substances such as house dust mites or animal hair (perennial allergic conjunctivitis)in adults and children from 2 years of age.
2. What you need to know before you use Bilaxten
Do not use Bilaxten
- if you are allergic to bilastine or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor or pharmacist before you start using Bilaxten if during treatment you experience side effects, such as eye irritation, pain, redness, or vision changes, or if your disease worsens. It may be necessary to interrupt treatment.
After administering the antiallergic eye drops of Bilaxten in the conjunctival sac of the eye, visual acuity may decrease for a few minutes due to the formation of spots.
In the case of inflammation, including allergic conjunctivitis, consult your ophthalmologist if you can use contact lenses despite the symptoms.
Children and adolescents
This medicine is indicated in adults and children from 2 years of age.
Do not administer this medicine to children under 2 years of age because its efficacy and safety have not been studied in these groups.
Other medicines and Bilaxten
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
If you are using other eye medicines, leave at least 5 minutes between each medicine.
Ophthalmic ointments should be administered last.
Pregnancy, breastfeeding, and fertility
Bilaxten can be used during pregnancy and breastfeeding. If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines
After instillation of this medicine, temporary blurred vision or other visual disturbances may occur that affect the ability to drive or use machines. Wait until your vision is clear before driving or using machinery.
Contact lenses
The use of this medicine does not affect the properties of contact lenses. You can continue to use contact lenses during the use of this medicine.
You must remove your contact lenses before applying the eye drops and not put them back until 15 minutes after administration.
3. How to use Bilaxten
Follow exactly the administration instructions of this medicine indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
The recommended dose for adults and children from 2 years of age is one drop in each affected eye once a day.
This medicine can be used for up to a maximum of 8 weeks. Your doctor will decide and advise you on how long you should use it based on your situation.
For ocular use only.
Method of administration
- Always wash your hands and dry them with a clean towel before administering this medicine.
- Gently clean your eyelids if there is discharge by rubbing the eyelid with the eye closed from the inner to the outer part with a cotton swab moistened with warm water.
- Open the bottle and avoid contact of the dropper tip with your eye or anything else: the drops and droppers must be kept clean.
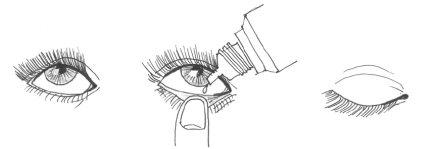
- Lean your head back, or lie down, and look up (Figure 1). Using your finger, pull the lower eyelid down (Figure 2).
- Look up and squeeze to release one drop into the eye.
- Release the lower eyelid and keep the eye closed for a while to spread the drop over the surface of the eye (Figure 3).
- Repeat the above action in the other eye if necessary.

To avoid contamination during the use of this medicine, do not touch any surface (eyelids, areas around the eye, or other surfaces) with the dropper tip and wipe the dropper tip with a clean paper towel after use to remove any residual liquid.
If you use more Bilaxten than you should
You can rinse with warm water. In case of doubt, consult your doctor. Also, in case of overdose or accidental ingestion, you can consult the Toxicology Information Service Tel.: 91 562 04 20.
If you forget to use Bilaxten
Do not use a double dose to make up for forgotten doses.
If you forget to apply the drop on time, apply the forgotten drop as soon as possible and then return to your usual dosing schedule.
If you stop using Bilaxten
Treatment with this medicine should be carried out regularly, if possible, until the symptoms are relieved. If you stop using Bilaxten while you are still exposed to allergens, you can expect the typical symptoms of the allergy to return.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been reported.
Uncommon side effects (may affect up to 1 in 100 people):
Altered taste (dysgeusia), headache.
Dry eye, eye discharge, eye irritation, increased tear production, eye discomfort.
If you experience any of the side effects described above, stop using this medicine and consult your doctor directly. The side effects mentioned are usually mild and disappear quickly in all cases. Therefore, no specific measures are required.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are possible side effects not listed in this package leaflet. You can also report them directly through the Spanish Medicines Monitoring System: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Bilaxten
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the label or carton after EXP. The expiry date is the last day of the month stated.
This medicine does not require any special storage conditions.
After opening the bottle:do not use this medicine if the bottle has been open for more than 2 months.
Medicines should not be disposed of via wastewater or household waste. Place the empty packaging and any unused medicine in the pharmacy's SIGRE collection point. If you have any doubts, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and other information
Composition of Bilaxten
- The active substance is bilastine 6 mg/ml.
One drop contains 0.2 mg of bilastine.
- The other ingredients are hydroxypropyl betadex, methylcellulose, sodium hyaluronate, glycerol (E 422), sodium hydroxide 1 N (for pH adjustment), water for injections.
Appearance and pack size
Bilaxten is a clear, colorless eye drop solution, contained in 1 multidose LDPE white bottle with 5 ml of preservative-free solution, with an HDPE white dropper and a tamper-evident security system.
Pack size: 1 bottle of 5 ml.
Marketing authorisation holder and manufacturer
Marketing authorisation holder
Faes Farma, S.A.
Autonomia Etorbidea, 10
48940 Leioa (Bizkaia)
Spain
Manufacturer
FAMAR Health Care Services Madrid, S.A.U.
Avenida Leganés, 62
28923 Alcorcón
Madrid
Spain
This medicine is authorised in the Member States of the European Economic Area under the following names:
Germany: Antires 6 mg/ml Augentropfen, Lösung;
Spain: Bilaxten 6 mg/ml colirio en solución;
France: Inorial 6 mg/ml collyre en solution;
Greece: Bilargen;
Italy: Robilas 6 mg/ml collirio, soluzione;
Poland: Bilaxten;
Portugal: Bilaxten 6 mg/ml colírio, solução;
Date of last revision of this package leaflet: June2025
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/).
- Country of registration
- Average pharmacy price18.8 EUR
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BILAXTEN 6 mg/ml EYE DROPS SOLUTIONDosage form: EYEDROP, 6 mg/mlActive substance: bilastineManufacturer: Menarini International Operations Luxembourg S.A.Prescription requiredDosage form: EYEDROP, 0.5 mg/mlActive substance: azelastineManufacturer: Cooper Consumer Health B.V.Prescription requiredDosage form: EYEDROP, 1 mg/mlActive substance: olopatadineManufacturer: Tiedra Farmaceutica S.L.Prescription required
Online doctors for BILAXTEN 6 mg/ml EYE DROPS SOLUTION
Discuss questions about BILAXTEN 6 mg/ml EYE DROPS SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















