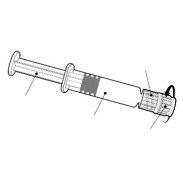
BEXSERO Injectable Suspension in Pre-filled Syringe

How to use BEXSERO Injectable Suspension in Pre-filled Syringe
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Bexseroinjection suspension in pre-filled syringe
Meningococcal group B vaccine (rDNA, component, adsorbed)
Read all of this leaflet carefully before you or your child receive this medicine, because it contains important information for you or your child.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- This vaccine has been prescribed for you or your child only.
- If you experience any side effects, talk to your doctor or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the pack and other information
- What is Bexsero and what is it used for
- What you need to know before you or your child receive Bexsero
- How to use Bexsero
- Possible side effects
- Storage of Bexsero
- Contents of the pack and further information
1. What is BEXSERO and what is it used for
Bexsero is a meningococcal group B vaccine.
Bexsero contains four different components from the surface of the Neisseria meningitidisgroup B bacteria.
Bexsero is given to individuals from 2 months of age to help protect them against diseases caused by Neisseria meningitidisgroup B bacteria. These bacteria can cause serious infections that can sometimes be life-threatening, such as meningitis (inflammation of the membrane that surrounds the brain and spinal cord) and sepsis (blood infection).
The vaccine works by specifically stimulating the body's natural defense system. This produces protection against the disease.
2. What you need to know before you or your child receive BEXSERO
Do not use Bexsero
- If you or your child are allergic to the active substances or any of the other components of this vaccine (listed in section 6).
Warnings and precautions
Talk to your doctor or nurse before you or your child receive Bexsero:
- If you or your child have a severe infection with high fever. In this case, vaccination will be postponed. The presence of a minor infection, such as a cold, is not a reason to postpone vaccination, but consult your doctor or nurse first.
- If you or your child have haemophilia or other problems that may affect blood clotting, such as treatment with anticoagulants. Consult your doctor or nurse first.
- If you or your child are receiving treatment that blocks the part of the immune system known as complement activation, such as eculizumab. Even if you or your child have been vaccinated with Bexsero, you or your child are still at higher risk of invasive disease caused by Neisseria meningitidisgroup B bacteria.
- If your child was born prematurely (at or before 28 weeks of gestation), especially if they had breathing difficulties. Stopping breathing or irregular breathing for a short period may be more common in the first three days after vaccination in these babies and may require special monitoring.
- If you or your child are allergic to the antibiotic kanamycin. If this is the case, the level of kanamycin in the vaccine is low. If you or your child may be allergic to kanamycin, consult your doctor or nurse first.
Fainting, feeling of loss of consciousness or other stress-related reactions can occur in response to any injection with a needle. Tell your doctor or nurse if you have had such a reaction in the past.
There are no data on the use of Bexsero in adults over 50 years of age. Data on the use of Bexsero in patients with chronic medical conditions or weakened immune systems are limited. If you or your child have a weakened immune system (for example, due to immunosuppressive drugs, HIV infection, or congenital defects of the body's natural defense system), the effectiveness of Bexsero may be reduced.
Like any vaccine, Bexsero may not fully protect all people who are vaccinated.
Using Bexsero with other medicines
Tell your doctor or nurse if you or your child are taking, have recently taken, or might take any other medicines or have recently received any other vaccine.
Bexsero can be given at the same time as any of the following vaccine components: diphtheria, tetanus, pertussis, Haemophilus influenzaetype b, poliomyelitis, hepatitis B, pneumococcal, measles, mumps, rubella, and varicella, and meningococcal A, C, W, Y. For more information, consult your doctor or nurse.
When given at the same time as other vaccines, Bexsero should be administered in separate injection sites.
Your doctor or nurse may ask you to give your child medicines that reduce fever at the time of Bexsero administration and afterwards. This will help reduce the side effects of Bexsero.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before receiving Bexsero. Your doctor may still recommend that you receive Bexsero if you are at risk of exposure to meningococcal infection.
Driving and using machines
Bexsero has no or negligible influence on the ability to drive and use machines. However, some of the effects mentioned in section 4 "Possible side effects" may temporarily affect the ability to drive or use machines.
Bexsero contains sodium chloride
This medicine contains less than 1 mmol sodium (23 mg) per dose, i.e., essentially "sodium-free".
3. How to use BEXSERO
Bexsero (0.5 ml) will be given to you or your child by a doctor or nurse. It will be injected into a muscle, usually in the thigh in infants or in the upper arm in children, adolescents, and adults.
It is important that you follow the doctor's or nurse's instructions to complete the series of injections.
Infants from 2 months up to 5 months of age at the time of the first dose
Your child should receive an initial series of two or three injections of the vaccine followed by a booster dose.
- The first injection should not be given before 2 months of age.
- If three initial doses are given, the interval between injections should be at least 1 month.
- If two initial doses are given, the interval between injections should be at least 2 months.
- A booster dose will be given between 12 and 15 months of age, after an interval of at least 6 months from the last injection of the initial series. If there is a delay in administration, the booster dose should not be given later than 24 months of age.
Infants from 6 to 11 months of age at the time of the first dose
Infants from 6 to 11 months of age should receive two injections of the vaccine followed by a booster dose.
- The interval between each injection should be at least 2 months.
- A booster dose will be given in the second year of life, after an interval of at least 2 months from the second injection.
Children from 12 months to 23 months of age at the time of the first dose
Children from 12 to 23 months of age should receive two injections of the vaccine, followed by a booster dose.
- The interval between each injection should be at least 2 months.
- A booster dose will be given after an interval of 12 to 23 months from the second injection.
Children from 2 to 10 years of age at the time of the first dose
Children from 2 to 10 years of age should receive two injections of the vaccine.
- The interval between each injection should be at least 1 month.
- Your child may receive a booster dose.
Adolescents and adults from 11 years of age at the time of the first dose
Adolescents (from 11 years of age) and adults should receive two injections of the vaccine.
- The interval between each injection should be at least 1 month.
- You may receive a booster dose.
Adults over 50 years of age
There are no data on adults over 50 years of age. Ask your doctor if Bexsero administration would be beneficial for you.
If you have any further questions on Bexsero, ask your doctor or nurse.
4. Possible side effects
Like all vaccines, this vaccine can cause side effects, although not everybody gets them.
When Bexsero is given to you or your child, the very common side effects (may affect more than 1 in 10 people) that you or your child may have are (observed in all age groups):
- Pain/tenderness at the injection site, redness of the skin at the injection site, swelling of the skin at the injection site, hardness of the skin at the injection site.
The following side effects may also occur.
Infants and children (up to 10 years of age)
Very common(may affect more than 1 in 10 people): fever (≥ 38 °C), loss of appetite, pain/tenderness at the injection site (including severe pain at the injection site that causes crying when the limb is moved), painful joints, skin rash (children from 12 to 23 months of age) (uncommon after booster dose), sleepiness, irritability, unusual crying, vomiting (uncommon after booster dose), diarrhoea, headache.
Common(may affect up to 1 in 10 people): skin rash (infants and children from 2 to 10 years of age)
Uncommon(may affect up to 1 in 100 people): high fever (≥ 40 °C), convulsions (including febrile convulsions), dry skin, paleness (rare after booster dose)
Rare(may affect up to 1 in 1,000 people): Kawasaki disease, which may include symptoms such as fever lasting more than five days, associated with skin rash on the trunk and sometimes followed by peeling of the skin on hands and feet, swollen lymph nodes in the neck, and redness of the eyes, lips, throat, and tongue. Itchy rash, skin rash
Adolescents (from 11 years of age) and adults
Very common(may affect more than 1 in 10 people): pain at the injection site that prevents normal daily activity, pain in muscles and joints, nausea, general feeling of being unwell, headache.
The following side effects have been reported during commercial use:
Swollen lymph nodes.
Allergic reactions, which may include severe swelling of the lips, mouth, throat (which may cause difficulty swallowing), difficulty breathing with wheezing (whistling sound while breathing) or coughing, rash, loss of consciousness, and very low blood pressure.
Fainting (sudden muscle weakness with loss of consciousness), decreased response to stimuli or loss of consciousness, and paleness or bluish discoloration of the skin in young children.
Feeling of loss of consciousness or fainting.
Skin rash (adolescents from 11 years of age and adults).
Fever (adolescents from 11 years of age and adults).
Injection site reactions such as extensive swelling of the vaccinated limb, blisters at the injection site or in the surrounding area, and hard lump at the injection site (which may last more than a month). Rarely, shortly after vaccination, stiffness of the neck or excessive sensitivity to light (photophobia) indicative of meningeal irritation have been reported; these symptoms have been mild and transient.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of BEXSERO
Keep this vaccine out of the sight and reach of children.
Do not use this vaccine after the expiry date which is stated on the carton and on the label of the pre-filled syringe after EXP. The expiry date is the last day of the month shown.
Store in a refrigerator (2 °C - 8 °C). Do not freeze.
Store in the original package to protect from light.
Medicines should not be disposed of via wastewater or household waste. Ask your doctor or nurse how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Container contents and additional information
Bexsero composition
A dose (0.5 ml) contains:
Active ingredients
Recombinant fusion protein NHBA 1,2,3 of Neisseria meningitidisgroup B | 50 micrograms |
Recombinant protein NadA 1,2,3 of Neisseria meningitidisgroup B | 50 micrograms |
Recombinant fusion protein fHbp 1,2,3 of Neisseria meningitidisgroup B | 50 micrograms |
Outer membrane vesicles (OMV) of Neisseria meningitidisgroup B strain NZ98/254 measured as the total amount of protein containing PorA P1.4 | 25 micrograms |
1 produced in E. colicells using recombinant DNA technology.
2 adsorbed on aluminum hydroxide (0.5 mg Al3+).
3 NHBA (Neisseria heparin-binding antigen), NadA (Neisseria adhesin A), fHbp (factor H-binding protein)
The other components:
Sodium chloride, histidine, sucrose, and water for injectable preparations (see section 2 for more information on sodium).
Product appearance andcontainer contents
Bexsero is a white opalescent suspension.
Bexsero is available in a pre-filled syringe with or without separate needles; pack sizes of 1 and 10.
Only some pack sizes may be marketed.
Marketing authorization holder:
GSK Vaccines S.r.l.
Via Fiorentina 1, 53100 Siena, Italy.
Manufacturer:
GSK Vaccines S.r.l.
Bellaria-Rosia, 53018 Sovicille (Siena), Italy.
For more information about this medicinal product, please contact the local representative of the marketing authorization holder:
België/Belgique/Belgien GlaxoSmithKline Pharmaceuticals SA/NV Tel: + 32 10 85 52 00 | Lietuva GSK Vaccines S.r.l. Tel: +370 80000334 |
България GSK Vaccines S.r.l. Tel: +359 80018205 | Luxembourg/Luxemburg GlaxoSmithKline Pharmaceuticals SA/NV Tel: + 32 10 85 52 00 |
Ceská republika GlaxoSmithKline s.r.o. Tel: + 420 2 22 00 11 11 | Magyarország GSK Vaccines S.r.l. Tel: +36 80088309 |
Danmark GlaxoSmithKline Pharma A/S Tlf: + 45 36 35 91 00 | Malta GSK Vaccines S.r.l. Tel: +356 80065004 |
Deutschland GlaxoSmithKline GmbH & Co. KG Tel: +49 (0)89 36044 8701 | Nederland GlaxoSmithKline BV Tel: + 31 (0)33 2081100 |
Eesti GSK Vaccines S.r.l. Tel: +372 8002640 | Norge GlaxoSmithKline AS Tlf: + 47 22 70 20 00 |
Ελλάδα GlaxoSmithKline Μονοπρόσωπη A.E.B.E. Τηλ: + 30 210 68 82 100 | Österreich GlaxoSmithKline Pharma GmbH. Tel: + 43 (0)1 97075 0 |
España GlaxoSmithKline, S.A. Tel: + 34 900 202 700 | Polska GSK Services Sp. z o.o. Tel: + 48 (22) 576 9000 |
France Laboratoire GlaxoSmithKline Tél: + 33 (0) 1 39 17 84 44 | Portugal GlaxoSmithKline – Produtos Farmacêuticos, Lda. Tel: + 351 21 412 95 00 |
Hrvatska GSK Vaccines S.r.l. Tel: +385 800787089 | România GSK Vaccines S.r.l. Tel: +40 800672524 |
Ireland GlaxoSmithKline (Ireland) Ltd Tel: + 353 (0)1 495 5000 | Slovenija GSK Vaccines S.r.l. Tel: +386 80688869 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika GSK Vaccines S.r.l. Tel: +421 800500589 |
Italia GlaxoSmithKline S.p.A. Tel: +39 (0)45 7741 111 | Suomi/Finland GlaxoSmithKline Oy Puh/Tel: + 358 10 30 30 30 |
Κύπρος GSK Vaccines S.r.l. Τηλ: +357 80070017 | Sverige GlaxoSmithKline AB Tel: + 46 (0)8 638 93 00 |
Latvija GSK Vaccines S.r.l. Tel: +371 80205045 | United Kingdom (Northern Ireland) GSK Vaccines S.r.l. Tel: + 44(0) 800 221441 |
Date of last revision of thisleaflet:
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
This information is intended solely forhealthcare professionals:
During storage, a fine white deposit may be observed in the suspension of the pre-filled syringe.
Shake the vaccine well before use to form a homogeneous suspension.
The vaccine should be visually inspected for particles or discoloration before administration.
In the event of foreign particles and/or alteration of the physical appearance, do not administer the vaccine. If the container contains two needles of different lengths, choose the most suitable one to ensure that the vaccine can be administered intramuscularly.
Do not freeze.
Bexsero should not be mixed with other vaccines in the same syringe.
If it is necessary to administer it simultaneously with other vaccines, it should be done in separate injection areas.
Ensure that the vaccine is injected intramuscularly only.
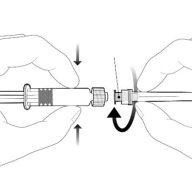
Instructions for the pre-filled syringe
| Hold the syringe by the body, not by the plunger. Unscrew the syringe cap by turning it counterclockwise. |
| To insert the needle, connect the base to the Luer-Lock adapter and turn it a quarter turn clockwise until it clicks. Do not remove the plunger from the syringe body. If this happens, do not administer the vaccine. |
Disposal of waste
Disposal of unused medicinal products and all materials that have come into contact with them should be carried out in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BEXSERO Injectable Suspension in Pre-filled SyringeDosage form: INJECTABLE, 0.5 ml single doseActive substance: meningococcus B, multicomponent vaccineManufacturer: Glaxosmithkline Vaccines S.R.L.Prescription requiredDosage form: INJECTABLE, 120 micrograms + 120 microgramsActive substance: meningococcus B, multicomponent vaccineManufacturer: Pfizer Europe Ma EeigPrescription requiredDosage form: INJECTABLE, 0.5 mlManufacturer: Sanofi Winthrop IndustriePrescription required
Online doctors for BEXSERO Injectable Suspension in Pre-filled Syringe
Discuss questions about BEXSERO Injectable Suspension in Pre-filled Syringe, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions