
BERINERT 500 IU POWDER AND SOLVENT FOR INJECTION AND INFUSION SOLUTION

How to use BERINERT 500 IU POWDER AND SOLVENT FOR INJECTION AND INFUSION SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Berinert 500 UI
Powder and solvent for solution for injection and infusion.
Human C1 esterase inhibitor
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Berinert and what is it used for
- What you need to know before you use Berinert
- How to use Berinert
- Possible side effects
- Storage of Berinert
- Contents of the pack and other information
1. What is Berinert and what is it used for
What is Berinert?
Berinert is presented as a powder and solvent. The prepared solution is to be administered by injection or infusion into a vein.
Berinert is prepared from human plasma (the liquid part of the blood), the active substance is human C1 esterase inhibitor protein, obtained from plasma.
What is Berinert used for?
Berinert is used for the treatment and preoperative prevention of hereditary angioedema type I and II (HAE, edema = swelling). Hereditary angioedema is a congenital vascular system disease. HAE is not an allergic disease. HAE is caused by insufficient, absent, or defective synthesis of the C1 esterase inhibitor, which is an important protein. The disease is characterized by the following symptoms:
- sudden swelling of hands and feet,
- sudden swelling of the face with a feeling of tightness,
- swelling of the eyelids, lips, possible swelling of the larynx (voice organ) with breathing difficulties,
- swelling of the tongue,
- colic-like pain in the abdominal region.
Generally, all parts of the body can be affected.
2. What you need to know before you use Berinert
The following sections contain information that your doctor should consider before administering Berinert to you.
Do not use Berinert:
- If you are allergic to C1 esterase inhibitor or to any of the other components of this medicine (listed in section 6).
Tell your doctor or pharmacist if you are allergic to any medicine or food.
Warnings and precautions
- If you have suffered allergic reactions to Berinert in the past. You should take antihistamines and corticosteroids as prophylaxis, if advised by your doctor.
- When allergic reactions or anaphylactic reactions (severe allergic reaction causing severe breathing difficulties or dizziness) occur. Berinert administration should be interrupted immediately (e.g., by interrupting the infusion).
- If you suffer from laryngeal swelling (laryngeal edema). You should be closely monitored and have emergency treatment ready for use.
- During use for the treatment of indications and dosages for which the medicine has not been approved (e.g., Capillary Leak Syndrome,CLS). See section 4 "Possible side effects".
Your doctor will carefully weigh the benefit of treatment with Berinert against the risk of these complications.
Viral safety
When administering medicines derived from human blood or plasma, certain measures must be taken to prevent infections from being passed on to patients. These measures include:
- careful selection of blood and plasma donors to exclude those who are at risk of being carriers of infectious diseases, and
- testing for specific virus and infection markers in individual donations and plasma pools,
Manufacturers of these products also include stages in the processing of blood or plasma to eliminate or inactivate viruses. Despite this, when administering medicines derived from human blood or plasma, the possibility of transmitting infectious agents cannot be entirely excluded. This also applies to emerging or unknown viruses or other types of infections.
The measures taken are considered effective for enveloped viruses such as human immunodeficiency virus (HIV, the AIDS virus), hepatitis B virus, hepatitis C virus (inflammation of the liver), and for non-enveloped viruses such as hepatitis A virus (inflammation of the liver) and parvovirus B19.
It is possible that your doctor may recommend vaccination against hepatitis A and B if you are treated periodically/repeatedly with human plasma-derived medicines.
It is strongly recommended that each time Berinert is administered, your doctor records the date of administration, batch number, and volume injected.
Using Berinert with other medicines
- Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines, including those without a prescription.
- Berinert must not be mixed with other medicines and diluents in the same syringe or infusion equipment.
Pregnancy and breastfeeding
- If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
- During pregnancy and breastfeeding, Berinert should only be used if clearly indicated.
Driving and using machines
The influence of Berinert on the ability to drive and use machines is negligible.
Berinert contains sodium
Berinert contains up to 49 mg of sodium (main component of cooking/table salt) per vial. This is equivalent to 2.5% of the maximum recommended daily sodium intake for an adult.
3. How to use Berinert
Treatment should be initiated and supervised by a doctor with experience in the treatment of C1 esterase inhibitor deficiency.
Dosage
Adults
Treatment of acute angioedema attacks:
20 UI per kilogram of body weight (20 UI/kg body weight).
Preoperative prevention of acute angioedema attacks:
1000 UI before 6 hours of a medical, dental, or surgical procedure.
Pediatric population
Treatment of acute angioedema attacks:
20 UI per kilogram of body weight (20 UI/kg body weight).
Preoperative prevention of acute angioedema attacks:
15 to 30 UI per kilogram of body weight (15-30 UI/kg body weight) before 6 hours of a medical, dental, or surgical procedure. The dose should be chosen taking into account the clinical circumstances (e.g., the type of procedure and the severity of the disease).
If you use more Berinert than you should
There have been no reports of overdose.
Reconstitution and method of administration
Berinert is injected into a vein (intravenous administration), usually by your doctor or nurse. You or your caregiver can also inject Berinert, but only after receiving adequate training. If your doctor decides that you can treat yourself at home, they will give you detailed instructions. You will be given a diary to record each injection administered at home, which you should bring with you each time you visit the doctor. You or your caregiver will be regularly reviewed to ensure that you are administering the injections correctly over time.
General instructions
- The powder must be dissolved and withdrawn from the vial under aseptic conditions. Use the syringe provided with the product.
- The prepared solution must be clear and transparent. After filtering or transferring the solution (see below), it should be visually checked for particles or discoloration before administration.
- Do not use the solution if it is visibly cloudy or contains particles or residues.
- Any unused medicine or material should be disposed of according to local regulations and your doctor's instructions.
Reconstitution
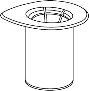
Before opening any vial, allow the Berinert powder and solvent to reach room temperature. To do this, you can leave the vials at room temperature for about an hour or hold them in your closed hands for a few minutes. DO NOT expose the vials to direct heat. The vials should not be heated to a temperature above body temperature (37°C).
Carefully remove the protective caps from the solvent vial and the powder vial. Clean the exposed rubber stoppers of both vials with an alcohol-impregnated swab and let them dry. You can now transfer the solvent to the powder vial using the included administration system (Mix2Vial). Please follow the instructions below:
|
|
|
|
|
|
|
|
|
Discard the solvent vial with the blue Mix2Vial adapter attached. |
|
|
|
|
Transfer of the reconstituted solution to the syringe and administration
8 |
|
9 |
|
Administration
The solution should be administered intravenously (i.v.) slowly, either by injection or infusion (4 ml/minute).
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Immediately contact your doctor
- if you experience any side effect or
- if you notice any side effect not mentioned in this leaflet.
Side effects with Berinert are rare.
The following side effects have been observed rarely (between 1 and 10 in 10,000 people/patients):
? There is an increased risk of thrombosis (blood clot formation) in attempts to treat or prevent Capillary Leak Syndrome (CLS), e.g., during and after cardiac surgery with extracorporeal circulation. See section 2 "Warnings and precautions".
- Increased body temperature, as well as burning and itching at the injection site.
- Hypersensitivity or allergic reactions (such as irregular heartbeat, rapid heartbeat, decreased blood pressure, skin redness, rash, breathing difficulties, headache, dizziness, feeling sick).
In very rare cases (less than 1 in 10,000 patients/people or isolated cases), allergic reactions can lead to shock.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Berinert
- Keep this medicine out of the sight and reach of children.
- Do not use Berinert after the expiry date, which is stated on the label and carton.
- Do not store above 30°C.
- Do not freeze.
- Keep the container in the outer carton to protect from light.
- Berinert does not contain preservatives, so the prepared solution should be used immediately.
- If the prepared solution is not administered immediately, it should be used within 8 hours. The reconstituted product should only be stored in the vial.
6. Container contents and additional information
Berinert composition
The active ingredient is:
Human C1 esterase inhibitor (500 IU/vial; after reconstitution: 50 IU/ml)
For more information, see the section "This information is intended only for healthcare professionals".
The other components are:
Glycine, sodium chloride, sodium citrate.
Consult the last paragraph of section 2. "Berinert contains sodium".
Solvent:water for injectable preparations.
Appearance of the product and container contents
Berinert is presented as a white powder and is supplied with water for injectable preparations as a solvent.
The prepared solution must be transparent and clear.
Presentation
Box with 500 IU contains:
1 vial with powder (500 IU)
1 vial with 10 ml of water for injectable preparations
1 transfer device with 20/20 filter
Administration equipment (inner box):
1 single-use 10 ml syringe
1 venipuncture device
2 alcohol swabs
1 dressing
Marketing authorization holder and manufacturer
CSL Behring GmbH
Emil von Behring Strasse, 76
35041 Marburg, Germany
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
CSL Behring S.A.
c/ Tarragona 157, 18th floor
08014 Barcelona
Spain
This medication is authorized in the member states of the European Economic Areaand in the United Kingdom (Northern Ireland)with the following names:
Berinert 500 IU Powder and solvent for solution for injection/infusion Austria
Berinert 500 Belgium, Cyprus, Germany, Greece, Luxembourg, Poland, Portugal
Berinert 500, 500 IU Powder and solvent for solution for injection/infusion Bulgaria
Berinert 500 IU Czech Republic, Slovakia
Berinert Denmark, Italy
Berinert 500 IU, powder and solvent for injectable/perfusion solution Finland
Berinert 500 IU, powder and solvent for injectable/perfusion solution France
Berinert 500 NE powder and solvent for injectable or infusion solution Hungary
Berinert 500 IU powder and liquid for injection/infusion solution, solution Norway
Berinert 500 IU, powder and solvent for injectable/perfusion solution Romania
Berinert 500 i.e. powder and vehicle for injection or infusion solution Slovenia
Berinert 500 UI Powder and solvent for injectable and perfusion solution Spain
Berinert 500 IE, powder and liquid for injection/infusion solution, solution Sweden
Berinert 500 UI powder and solvent for solution for injection/infusion United Kingdom
Date of the last revision of this prospectus:October 2021
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
Thisinformation is intended only for healthcare professionals
QUALITATIVE AND QUANTITATIVE COMPOSITION
The potency of the human C1 esterase inhibitor is expressed in International Units (IU), which is related to the current WHO standard for C1 esterase inhibitor products.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BERINERT 500 IU POWDER AND SOLVENT FOR INJECTION AND INFUSION SOLUTIONDosage form: INJECTABLE, 1500 IUActive substance: c1-inhibitor, plasma derivedManufacturer: Csl Behring GmbhPrescription requiredDosage form: INJECTABLE, 2000 IUActive substance: c1-inhibitor, plasma derivedManufacturer: Csl Behring GmbhPrescription requiredDosage form: INJECTABLE, 3000 IUActive substance: c1-inhibitor, plasma derivedManufacturer: Csl Behring GmbhPrescription required
Online doctors for BERINERT 500 IU POWDER AND SOLVENT FOR INJECTION AND INFUSION SOLUTION
Discuss questions about BERINERT 500 IU POWDER AND SOLVENT FOR INJECTION AND INFUSION SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions




 1
1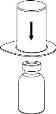 2
2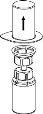 3
3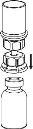 4
4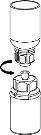 5
5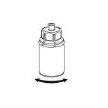 6
6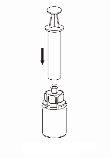 7
7










