
BELKYRA 10 mg/ml INJECTABLE SOLUTION

How to use BELKYRA 10 mg/ml INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
BELKYRA 10mg/ml solution for injection
deoxycholic acid
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is BELKYRA and what is it used for
- What you need to know before you use BELKYRA
- How to use BELKYRA
- Possible side effects
- Storage of BELKYRA
- Contents of the pack and other information
1. What is BELKYRA and what is it used for
Belkyra contains the active substance deoxycholic acid. The body produces deoxycholic acid naturally to help digest fats.
This medicine is used in adults for the treatment of submental fat (unwanted fat under the chin) when its presence has a significant psychological impact on the patient.
Belkyra contains a non-human and non-animal version of deoxycholic acid, which is identical to the deoxycholic acid produced naturally. Belkyra is an injectable medicine administered by your doctor.
2. What you need to know before you use BELKYRA
Do not use BELKYRA:
- If you are allergic to deoxycholic acid or any of the other ingredients of this medicine (listed in section 6).
- If you have an infection in the area of the chin or neck where the medicine is to be injected.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before starting treatment with Belkyra. Your doctor or nurse will check your health status before each treatment. Inform your doctor or nurse of any illness you suffer from before each treatment.
Your doctor or nurse will pay special attention to the area around your neck, as precautions need to be taken in case of any previous disease or surgery (e.g., scars, liposuction, difficulty swallowing, enlargement of the thyroid gland or lymph nodes).
- A temporary injury of the jaw nerve may occur, which can lead to an asymmetric smile or weakness of the facial muscles.
- Tissue damage around the treated area (i.e., skin erosion, ulceration, necrosis) may occur. This can cause scarring. If ulceration or necrosis occurs, you should never receive treatment with Belkyra again (see section 4).
- Infection around the treatment area may occur and may require additional medical treatment. If redness or pain occurs, talk to your doctor, pharmacist, or nurse.
Belkyra should not be used if you have obesity or body dysmorphic disorder (distorted perception of your own image).
Children and adolescents
This medicine is not intended for use in children and adolescents.
Other medicines and BELKYRA
Tell your doctor or nurse if you are taking, have recently taken, or might take any other medicines.
Pregnancy and breastfeeding
The effects of this medicine in pregnant or breastfeeding women are not known. As a precaution, the use of Belkyra is not recommended during pregnancy.
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or nurse for advice before using this medicine.
Driving and using machines
Belkyra is not expected to affect your ability to drive or use machines.
BELKYRA contains sodium
This medicine contains 4.23 mg of sodium (main component of cooking/table salt) in each ml. This is equivalent to 0.2% of the maximum daily recommended sodium intake for an adult.
3. How to use BELKYRA
How BELKYRA is administered
Belkyra is administered directly under the skin ("subcutaneously") by a doctor, or in countries where regulations allow, by a healthcare professional under the supervision of a doctor. Belkyra will be injected in small amounts into several points in the treatment area, which is the fatty tissue directly under the skin in the area under the chin.
Your doctor may take some measures to alleviate pain before and after injection.
Dose
Your doctor will decide the amount of Belkyra administered.
You will receive several injections per treatment session. The total number of injections and treatment sessions needed to achieve a satisfactory response will depend on your individual needs and will be decided by your doctor. Treatment may be repeated several times, but no more than 6 treatment sessions should be performed, with 2 to 4 sessions usually being sufficient. The time between treatment sessions should be at least 4 weeks.
If you have been given too much BELKYRA
If you have been given more Belkyra than recommended, local side effects may increase (see section 4). In this case, talk to your doctor or nurse.
At the end of this leaflet, more information is provided on the use and handling of the medicine by the doctor or healthcare professional.
If you have any other questions about the use of this medicine, ask your doctor or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
- A temporary injury of the jaw nerve may occur, which can lead to an asymmetric smile or weakness of the facial muscles.
- Tissue damage around the treated area (i.e., skin erosion, ulceration, necrosis) may occur. This can cause scarring.
If you experience any of the above side effects, tell your doctor or nurse immediately.
The following is a list of side effectsobserved with the following frequencies:
Very common(may affect more than 1 in 10 people):
- Reactions at the injection site:
- pain
- fluid retention in the tissue (edema) and swelling
- sensory symptoms (paresthesia): loss of sensation, reduced sensation, numbness, tingling, unusual sensation
- small, round area of localized hardness (nodule)
- bruises
- stiffness or thickening of the tissue (induration)
- redness of the skin (erythema)
- itching
Common(may affect up to 1 in 10 people):
- Reactions at the injection site:
- bleeding
- discomfort
- heat
- change in skin color
- Injury to the nerve around the jaw
- Tension in the skin
- Difficulty swallowing (dysphagia)
- Nausea
- Headache
Uncommon(may affect up to 1 in 100 people):
- Unusual taste in the mouth (dysgeusia)
- Difficulty speaking (dysphonia)
- Reactions at the injection site:
- hair loss (alopecia)
- hives
- skin lesions (ulcers)
- allergic reaction (hypersensitivity)
- scarring
Frequency not known(cannot be estimated from the available data)
- Reduced or abnormal sensation in the mouth area (e.g., lip, tongue) (oral hypoesthesia, oral paresthesia).
- Reaction at the injection site (see "Warnings and precautions"):
- decreased sensitivity to touch or altered sensation in the cheek.
- tissue damage and cell death (necrosis) around the treated area.
- Infection including redness, swelling, or pain (cellulitis) or a collection of pus (abscess)
- injury to blood vessels if accidentally injected into an artery or vein.
Most side effects observed improved during the 4-week period between treatments. However, some of these injection site reactions may last longer.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of BELKYRA
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after "EXP". The expiry date refers to the last day of the month shown.
This medicine does not require any special storage conditions. It is recommended to use the injection solution immediately after opening.
Do not use this medicine if you notice visible particles.
6. Contents of the pack and other information
Composition of BELKYRA
- The active substance is deoxycholic acid.
1 ml of injection solution contains 10 mg of deoxycholic acid. 1 vial with 2 ml contains 20 mg of deoxycholic acid.
- The other ingredients are water for injections, sodium chloride, sodium hydroxide (for dissolution and pH adjustment), hydrochloric acid (for pH adjustment), and disodium phosphate anhydrous.
Appearance of BELKYRA and contents of the pack
Belkyra is a clear, colorless, and sterile solution for injection.
Pack size:
One carton with 4 vials (Type I glass with a chlorobutyl rubber stopper, aluminum seal, and flip-off cap made of polypropylene).
Each vial contains 2 ml of injection solution.
Marketing authorization holder and manufacturer
Marketing authorization holder
AbbVie Spain, S.L.U.
Avenida de Burgos 91,
28050 Madrid
Spain
Manufacturer
Almac Pharma Services, Ltd.
Seagoe Industrial Estate,
Portadown,
Craigavon,
County Armagh, BT63 5QD
United Kingdom
Allergan Pharmaceuticals International Ltd.
Clonshaugh Business & Technology Park,
Dublin 17,
D17 E400,
Ireland
Date of last revision of this leaflet:
April 2023
Detailed and up-to-date information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
--------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
The injection solution should be visually inspected before use. Only clear, colorless, and particle-free solutions should be used.
Posology
The total volume injected and the number of treatment sessions should be tailored to the treatment objectives and the distribution of submental fat in each individual patient.
Inject 0.2 ml (2 mg) per injection point, with 1 cm distance. Do not exceed the maximum dose of 10 ml (100 mg, equivalent to 50 injections) in a treatment session.
A maximum of 6 treatment sessions may be performed, although most patients will show improvement between treatment sessions 2 and 4. The time interval between treatment sessions should be at least 4 weeks.
For the patient's comfort during injections, oral analgesics or NSAIDs, topical or injectable local anesthesia (e.g., lidocaine), and/or cold compresses with frozen gel packs may be administered to the injection site, at the discretion of the healthcare professional.
Method of administration
The product is intended for subcutaneous administration only.
Belkyra is supplied in single-use vials ready for use. Slowly invert the vial several times before use and do not dilute.
Belkyra should be prepared for injection as follows:
- Remove the flip-off cap from the vial and clean the vial stopper with an antiseptic. Do not use if the vial, seal, or cap is damaged.
- Place a large-caliber sterile needle on a 1 ml sterile disposable syringe.
- Insert the large-caliber sterile needle into the vial stopper and withdraw 1 ml of Belkyra into the 1 ml syringe.
- Replace the large-caliber needle with a 30 G (or smaller) 12-13 mm needle. Expel air bubbles from the syringe before injecting the product into the subcutaneous fat.
- To withdraw the remaining contents of the vial, repeat steps 3 and 4.
Belkyra should only be administered by doctors with training, experience in treatment, and adequate knowledge of submental anatomy. In countries where regulations allow, healthcare professionals with adequate training may administer Belkyra under the supervision of a doctor. The safe and effective use of Belkyra depends on the proper selection of patients, which includes knowledge of the patient's history of previous interventions and the possibility that these may have changed the cervical superficial anatomy. Particular caution should be exercised when using Belkyra in patients with excessive skin laxity, prominent platysmal bands, or other conditions in which a reduction of submental fat may produce undesirable results.
Insert the needle perpendicularly to the skin to inject Belkyra.
The placement of the needle in relation to the jaw is very important, as it reduces the possibility of injury to the marginal mandibular nerve, a motor branch of the facial nerve. This nerve injury presents as an asymmetric smile due to paresis of the lip depressor muscles.
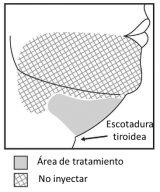
To avoid injury to the marginal mandibular nerve:
- Do not inject above the lower edge of the jaw.
- Do not inject in a region defined by a line 1-1.5 cm below the lower edge (from the angle of the jaw to the chin).
- Inject Belkyra only within the treatment area with target submental fat (see figures 1 and 3).
Figure 1. Avoid the marginal mandibular nerve area
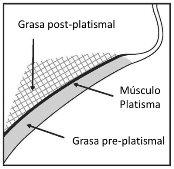
Avoid injecting into the platysma muscle. Before each treatment session, palpate the submental area to ensure the presence of sufficient submental fat and to identify the subcutaneous fat between the dermis and the platysma muscle (preplatysmal fat) in the target treatment area (figure 2).
Figure 2. Sagittal view of the platysmal area
Mark the planned treatment area with a surgical pencil and use a 1 cm2 template to mark the injection points (figures 2 and 3).
Figure 3. Treatment area and injection pattern
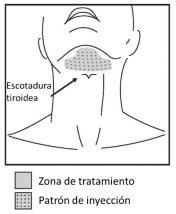
Do not inject Belkyra outside the defined parameters.
Each vial is for single-patient use. After use, discard any unused medicine.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BELKYRA 10 mg/ml INJECTABLE SOLUTIONDosage form: TABLET, 1 mgActive substance: finasterideManufacturer: Industrial Farmaceutica Cantabria S.A.Prescription requiredDosage form: TOPICAL SOLUTION, 2.275 mg/mlActive substance: finasterideManufacturer: Industrial Farmaceutica Cantabria S.A.Prescription requiredDosage form: TOPICAL SOLUTION, 50 mg/mlActive substance: minoxidilManufacturer: Mibe Pharma Espana S.L.Prescription not required
Online doctors for BELKYRA 10 mg/ml INJECTABLE SOLUTION
Discuss questions about BELKYRA 10 mg/ml INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions








