
ALOCARE 2.275 mg/ml CUTANEOUS SPRAY SOLUTION


How to use ALOCARE 2.275 mg/ml CUTANEOUS SPRAY SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: information for the patient
Alocare 2,275 mg/ml solution for cutaneous spray
finasteride
Read this leaflet carefully before starting to take this medicine, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any doubts, consult your doctor or pharmacist.
- This medicine has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the leaflet
- What is Alocare and what is it used for
- What you need to know before starting to use Alocare
- How to use Alocare
- Possible side effects
- Storage of Alocare
- Package contents and additional information
1. What is Alocare and what is it used for
This medicine contains the active ingredient finasteride. It is administered on the scalp of the bald skin through a spray applicator consisting of a bottle with a pump and a cone.
Alocare is used for the treatment of mild to moderate male hair loss (also known as androgenetic alopecia). This medicine increases hair growth and prevents further hair loss in men. Alocare can only be used in men between 18 and 41 years old.
Male-type hair loss is a common disorder that is believed to be caused by a combination of genetic factors and a particular hormone, called dihydrotestosterone (DHT).
It is believed that this hormone contributes to shortening the hair growth phase and making it thinner.
As the hair follicles become smaller, baldness becomes evident. Alocare reduces the level of DHT in the scalp. This helps to reverse the balding process, leading to increased hair growth and prevention of further hair loss.
2. What you need to know before starting to use Alocare
Do not use Alocare
- If you are allergic to finasteride or any of the other components of this medicine (listed in section 6).
- If you are a woman and are or may become pregnant.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Alocare.
Possible transmission of Alocare.
If the active ingredient is absorbed through the skin of a pregnant woman carrying a male baby, the baby may be born with abnormalities in his genital organs.
Avoid any contact between the treated area and a woman who is or may become pregnant. You should also avoid touching surfaces exposed to this medicine. If contact with Alocare occurs, the affected woman should wash the affected area quickly and thoroughly.
Children and adolescents should not come into contact with this medicine. If contact with Alocare occurs, the affected child or adolescent should wash the affected area quickly and thoroughly.
Effects on Prostate-Specific Antigen (PSA)
If you are going to have a blood test for prostate-specific antigen (PSA) for prostate cancer detection, inform your doctor that you are using Alocare, as it may affect the interpretation of the results.
Effect on the male hormone dihydrotestosterone (DHT)
Alocare decreases the concentration of the male hormone (DHT) in blood. However, this occurs rarely and the decrease is less than with finasteride tablets. The secondary sexual effects known for finasteride tablets may also occur with Alocare but are less likely (see section 4). Therefore, follow the dose prescribed by your doctor. Do not use more than 4 sprays per day.
Breast cancer
Although breast cancer has not been detected in men treated with Alocare in clinical studies, it has been reported during treatment with finasteride tablets. If you experience any changes in breast tissue, such as lumps, pain, breast tissue increase, or nipple discharge, contact your doctor as soon as possible.
Mood changes and depression
Although mood changes have not been observed in patients treated with Alocare in clinical studies, they have been reported during treatment with finasteride tablets. If you experience symptoms such as depressed mood, depression, or suicidal thoughts, contact your doctor for advice as soon as possible.
Children and adolescents
This medicine should not be used in children or adolescents. There is no data demonstrating the efficacy and safety of finasteride in children under 18 years old.
Other medicines and Alocare
Inform your doctor or pharmacist if you are taking, have recently taken, or may need to take any other medicine.
Do not apply Alocare if you are using other topical products, such as cosmetics, sunscreens, or other medicines, on the same area.
Pregnancy
Women should not take Alocare.
Women who are or may become pregnant should avoid contact with the treated scalp or surfaces exposed to Alocare. See the section "Possible transmission of Alocare" above. If direct contact with this medicine occurs, the woman should wash the affected area quickly and thoroughly and seek advice from her doctor.
Driving and using machines
Alocare does not affect the ability to drive or operate machinery.
Alocare contains ethanol
This medicine contains 25 mg of ethanol (96%) per spray, which is equivalent to 0.5 mg/microliter (55%). It may cause a burning sensation on damaged skin.
3. How to use Alocare
Follow the administration instructions of this medicine exactly as indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
Depending on the extent of your bald scalp, your doctor will prescribe between 1 and 4 sprays per day to be used in different areas. Do not use more than 4 sprays per day.
This medicine is for cutaneous use only. It should only be used on the scalp.
Alocare consists of two separate components: a bottle with a pump attached and a cone. These components require assembly before the first use. Before the first application, read all the instructions indicated below.
Make sure your hair and scalp are completely dry before application. Apply Alocare to the scalp by itself. If more than one spray is prescribed, apply them to non-overlapping areas. Do not apply the solution to other areas of the body except the scalp. Once applied, let Alocare act for at least 6 hours.
This medicine can be transferred by contact with fabrics, hands, or other surfaces and objects. Avoid contact between the treated scalp and pillows, helmets, hats, etc. until the solution has dried.
Alocare can be transferred from your body to others if they touch your treated scalp or other exposed surfaces. If contact with Alocare occurs, the person should wash the affected area quickly and thoroughly.
Keep Alocare in a safe place out of the reach of children. Warn family members or other people with access to the storage area about the contact precautions.
Components and assembly of the spray
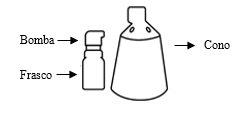
Assembly of the spray
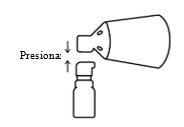 Align and press
Align and press
Align the cone with the pump button and press firmly.
- Correct assembly
 The spray applicator is correctly assembled when you hear a click after pressing, the spray nozzle is in the central position of the cone
The spray applicator is correctly assembled when you hear a click after pressing, the spray nozzle is in the central position of the cone
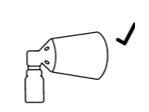 Correct assembly
Correct assembly
and the lower part of the pump button is aligned with the lower part of the cone without separation.
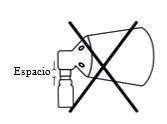 Incorrect assembly if there is a gap. Realign and press again
Incorrect assembly if there is a gap. Realign and press again
If you do not hear a click during assembly or see a gap between the lower part of the pump button and the cone, realign the components and press them into place again.
Preparing the pump
- After assembling the spray applicator, you need to prepare the pump for the first use. If the spray applicator has not been used for 2 weeks or more, you will need to prepare the pump again. It is not necessary to prepare it for each use.
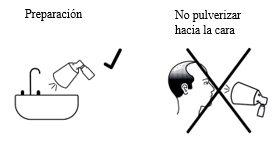
- To prepare the pump for the first time, press the pump all the way down four times with your thumb or index finger, directing the sprayed solution to a sink. Then, rinse the sink with water. To prepare the pump again after not using it for 2 weeks or more, press the pump all the way down once.
- Do not spray Alocare towards your face.
If solution is released during assembly or preparation, clean the surfaces where it may have been deposited
Dose application
- Depending on the size of the bald area of the scalp, your doctor will prescribe between 1 and 4 sprays per day.
- It is not necessary to shake the bottle before use.
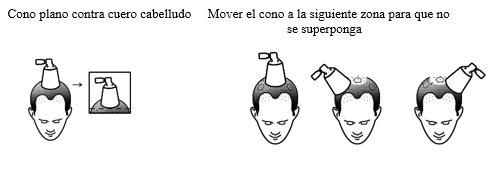
- Hold the spray applicator with the cone flat against the scalp to avoid dispersing the solution in the air.
- Press the pump all the way down once for one spray.
- Move the cone to different areas of the scalp to apply other doses according to the number of sprays prescribed by your doctor. Do not overlap and treat areas that have already been sprayed.
- After use, do not remove the cone from the pump. Put the spray applicator back in the box.
- Once applied, do not wash Alocare for at least 6 hours.
Make sure Alocare does not come into contact with your hands or any other part of your body. Immediately and thoroughly wash any exposed area that is not your scalp.
If the cone becomes soiled, clean it with a clean and dry paper towel. Safely dispose of the used paper towel and wash your hands well.
Dose and treatment days per dose
The bottle contains up to 180 sprays. The number of treatment days depends on the prescribed dose, from 1 to 4 sprays per day. Do not use the bottle beyond 180 sprays, as the remaining solution in the bottle may not provide a complete dose, which could limit the effect of your treatment.
Sprays per day | Treatment days |
1 | 180 |
2 | 90 |
3 | 60 |
4 | 45 |
- The pharmacist will note on the box the prescribed dose and the remaining number of treatment days until the product is depleted.
- On the start date of treatment with Alocare, note on your calendar the prescribed dose (from 1 to 4 sprays) and calculate when you will need a new bottle. Contact your doctor before your supply runs out so that there is no interruption of treatment.
If you use more Alocare than you should
If you apply more Alocare than recommended, talk to your doctor. Alocare will not work faster or better if applied more than once a day, but side effects may occur more frequently.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount administered.
If you forget to take Alocare
If you forget to apply Alocare, do not apply a double dose to make up for the missed application. Continue using the dose recommended by your doctor.
If you interrupt treatment with Alocare
The effect of treatment may take 3 months to develop. It is important that you continue using Alocare for the time indicated by your doctor. If you stop applying Alocare, you will likely lose the hair you have gained.
If you have any other doubts about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everyone gets them.
Common (may affect up to 1 in 10 people)
- Itching or redness of the scalp
Very common (may affect more than 1 in 10 people)
- Decrease in a male hormone (dihydrotestosterone) in blood
Other side effects known with oral finasteride may also occur with Alocare.
This includes:
- Allergic reactions (hypersensitivity) including skin rash, itching, swelling around the mouth (angioedema)
- Depressed mood
- Anxiety
- Heartbeat sensation (palpitations)
- Increased liver enzymes
- Breast tenderness and increase
- Testicular pain
- Blood in the ejaculate (hematospermia)
- Decreased sexual desire
- Difficulty having an erection
- Ejaculation disorder, including decreased ejaculate volume
- Infertility
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the Spanish Medicines Monitoring System: https://www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Alocare
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the carton, after EXP. The expiration date is the last day of the month indicated.
This medicine does not require special storage conditions.
Alocare contains alcohol and is therefore flammable. Avoid spraying the medicine near open flames or while smoking.
Do not use Alocare for more than 6 months after opening the bottle for the first time.
Medicines should not be disposed of via wastewater or household waste. Deposit the containers and medicines you no longer need at the pharmacy's SIGRE point. If in doubt, ask your pharmacist how to dispose of the containers and medicines you no longer need. This will help protect the environment.
6. Container contents and additional information
Alocare composition
- The active ingredient is finasteride. Each mL of solution contains 2.275 mg of finasteride. Each spray provides 50 microliters, which contain 114 micrograms of finasteride.
- The other components are: ethanol (96%), purified water, propylene glycol, hydroxypropyl chitosan.
Product appearance and container contents
Alocare is a colorless, transparent, and slightly viscous cutaneous spray solution.
Container size:
- 1 bottle (corresponding to 180 sprays) with a mechanical pressure spray pump and 1 separate cone.
- 3 bottles (corresponding to 3 x 180 sprays) with a mechanical pressure spray pump and 3 separate cones.
Before the first use, attach the cone to the bottle pump, as described in section 3.
Only some container sizes may be marketed.
Marketing authorization holder
Industrial Farmacéutica Cantabria, S.A.
Ctra. Cazoña-Adarzo, s/n
39011 Santander
Spain
Manufacturer
Almirall Hermal GmbH
Scholtzstrasse 1 and 3- Reinbek
Schleswig-Holstein – 21465
Germany
This medicinal product is authorized in the EEA member states with the following names:
Country | Trade names |
Germany | Finjuve für Männer 2,275 mg/ml Spray zur Anwendung auf der Haut (Kopfhaut), Lösung |
Bulgaria | ?????? ?? ???? 2,275 mg/ml ????? ?? ????, ??????? Finjuve for men 2.275 mg/ml cutaneous spray, solution |
Czech Republic | Fynzur 2.275 mg/ml kožní sprej, roztok |
Hungary | Fynzur férfiaknak 2,275 mg/ml külsoleges oldatos spray |
Italy | CARETOPIC 2.275 mg/ml spray cutaneo, soluzione |
Luxembourg | Finjuve für Männer 2,275 mg/ml Spray zur Anwendung auf der Haut (Kopfhaut), Lösung |
Poland | Finjuve, 2,275 mg/ml, aerozol na skóre, roztwór |
Portugal | Finasterida Cantabria 2.275 mg/ml Solução para Pulverização Cutânea |
Romania | Finjuve pentru barba?i 2,275 mg/ml spray cutanat, solutie |
Slovakia | Finjuve pre mužov 2,275 mg/ml dermálny roztokový sprej |
Spain | Alocare 2,275 mg/ml Solución para pulverización cutánea |
Date of the last revision of this prospectus:February 2022
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ALOCARE 2.275 mg/ml CUTANEOUS SPRAY SOLUTIONDosage form: TABLET, 1 mgActive substance: finasterideManufacturer: Industrial Farmaceutica Cantabria S.A.Prescription requiredDosage form: TABLET, 1 mg / tabletActive substance: finasterideManufacturer: Aurovitas Spain, S.A.U.Prescription requiredDosage form: TABLET, 1 mgActive substance: finasterideManufacturer: Laboratoires Bailleul S.A.Prescription required
Online doctors for ALOCARE 2.275 mg/ml CUTANEOUS SPRAY SOLUTION
Discuss questions about ALOCARE 2.275 mg/ml CUTANEOUS SPRAY SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions








