
BECLO-RINO 50 micrograms Nasal Spray Suspension

How to use BECLO-RINO 50 micrograms Nasal Spray Suspension
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Beclo-Rino 50 micrograms/spray nasal suspension
Beclometasone dipropionate
Read this package leaflet carefully before you start using this medicine because it contains important information for you:
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is Beclo-Rino and what is it used for
- What you need to know before you use Beclo-Rino
- How to use Beclo-Rino
- Possible side effects
- Storage of Beclo-Rino
- Contents of the pack and further information
1. What is Beclo-Rino and what is it used for
Beclo-Rino is an aqueous suspension of beclometasone dipropionate for nasal spray.
It is a medicine that acts as an antiallergic.
It is indicated for the temporary relief of symptoms of allergic rhinitis caused by pollen from plants (hay fever), domestic animals, dust or other allergens (smoke, pollution), such as runny nose, nasal congestion, sneezing and itching of the nose.
It is also indicated in nasal polyps.
2. What you need to know before you use Beclo-Rino
Do not use Beclo-Rino:
- If you are allergic to beclometasone dipropionate or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions:
Talk to your doctor or pharmacist before you start using Beclo-Rino:
- If you have any infection in the nasal passages or pulmonary tuberculosis.
- If you experience pain or bleeding in the nasal passages, you should stop treatment.
- If you are using or have recently used oral or injectable corticosteroid medications.
- Be careful when inserting the applicator into the nose:if you have wounds or ulcers in the nasal passages, or if you have recently undergone nasal surgery.
- If you use this medicine for prolonged periods, your doctor will periodically review:
- The condition of your nasal mucosa
- The condition of your adrenal glands
- If Beclo-Rino is administered to children over 6 years of age, the doctor should periodically review their growth, as this medicine may cause growth retardation.
Contact your doctor if you experience blurred vision or other visual disturbances.
Children
Beclo-Rino should not be used in children under 6 years of age.
Using Beclo-Rino with other medicines:
Tell your doctor if you are taking or have recently taken other medicines, including those obtained without a prescription.
Some medicines may increase the effects of Beclo-Rino, so your doctor will monitor you closely if you are taking these medicines (including some for HIV: ritonavir, cobicistat).
Pregnancy and breastfeeding:
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Driving and using machines:
This medicine has no effects on the ability to drive or use machines.
Use in athletes:
Athletes should be aware that this medicine contains beclometasone dipropionate, which may produce a positive result in doping tests.
Beclo-Rino contains benzalkonium chloride and glucose:
This medicine may cause inflammation of the nasal mucosa, especially with long-term treatments, because it contains benzalkonium chloride. If such a reaction is suspected (persistent nasal congestion), whenever possible, a nasal medicine that does not contain this excipient should be used.
This medicine contains 5 mg of glucose per application, which should be taken into account by diabetic patients.
3. How to use Beclo-Rino
Follow exactly the administration instructions of this medicine indicated by your doctor or pharmacist. If in doubt, ask your doctor or pharmacist.
Beclo-Rino is a medicine that is administered nasally.
Children from 6 years of age: according to medical criteria.
Adults over 18 years of age: the recommended dose is 1 or 2 applications (50 micrograms of beclometasone dipropionate per application) in each nostril, as needed, twice a day.
Do not perform more than 4 applications per day (200 micrograms of beclometasone dipropionate) in each nostril.
Do not use for more than 7 consecutive days.
If you think the effect of Beclo-Rino is too strong or too weak, tell your doctor or pharmacist.
Guidelines for correct administration:
Check the expiration date of the product before use.
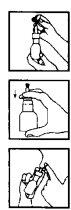 Before performing the first application of Beclo-Rino, you should:
Before performing the first application of Beclo-Rino, you should:
- Remove the plastic cap from the nasal applicator.
- Shake the bottle-applicator and press several times by pushing down the applicator, using the index and middle fingers, while holding the base of the container with the thumb. When you have a uniform spray (approximately 5 presses), the medicine is ready to be used. If not used daily, it is necessary to shake the container and perform a press in the air before using it again.
Before each application, you should:
- Blow your nose gently.
- Remove the plastic cap from the nasal applicator.
- Shake the bottle-applicator.
- Insert the applicator into one nostril, while covering the other by pressing laterally with your finger.
- Lean your head slightly forward and, while inhaling, press the applicator firmly on the bottom of the bottle. Breathe through your mouth and repeat the process.
- Repeat the same process with the other nostril.
After finishing the application, you should put the plastic cap back on the nasal applicator.
Cleaning:
It is recommended to regularly clean the plastic cap and the nasal applicator, washing them with warm water. Let them dry and put them back correctly.
If the nasal applicator becomes clogged, remove the cap, take out the nasal applicator and soak it in warm water for a few minutes. Rinse with cold water, dry and put it back in the bottle.
The nasal applicator is removed by gently pulling upwards.
If you use more Beclo-Rino than you should:
It is important that you take your dose as indicated by your doctor. You should only use what your doctor recommends, using more or less dose may worsen your symptoms.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service 91 562 04 20, indicating the medicine and the amount administered.
If you forget to use Beclo-Rino:
If you forget to apply a dose, apply it as soon as you remember. Do not use a double dose to make up for forgotten doses.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The side effects due to the nasal application of Beclo-Rino are generally mild and transient.
The following side effects have been reported:
- Very common (may affect more than 1 in 10 people): headache that may be caused by the allergic process or by the administration of the medicine.
- Common (may affect up to 1 in 10 people): sore throat, sneezing, tearing, nasal bleeding, cough, dryness, itching and burning in the nose, bad taste and mouth odor that may be caused by the allergic process or by the administration of the medicine, as well as nasopharyngeal candidiasis (white patches in the nose and throat).
- Very rare (may affect up to 1 in 10,000 people): allergies, which manifest with: rashes, itching, eczema, swelling of the eyelids, lips, face and throat, anaphylactic or anaphylactoid reactions (severe allergic reactions that can make breathing difficult or alter your level of consciousness) and sudden feeling of suffocation, as well as increased intraocular pressure, glaucoma, cataracts and perforation of the nasal septum.
- Frequency not known (cannot be estimated from the available data): blurred vision.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if it is possible side effects not listed in this leaflet. You can also report them directly through the Spanish Medicines Agency's website: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Beclo-Rino
Keep this medicine out of the sight and reach of children.
Do not store above 30°C. Store in the original package to protect from light. Do not refrigerate.
Do not use Beclo-Rino after the expiry date stated on the package after CAD. The expiry date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and further information
Composition of Beclo-Rino:
- The active substance is beclometasone dipropionate.
- The other ingredients (excipients) are: glucose, microcrystalline cellulose, sodium carboxymethylcellulose, polysorbate 80, benzalkonium chloride, phenylethanol and purified water.
Appearance of the product and pack contents:
Beclo-Rino is a white aqueous suspension and comes in a bottle with a dosing pump and nasal applicator.
Each bottle contains 200 applications.
Marketing authorisation holder and manufacturer:
Laboratorio ALDO-UNIÓN, S.L.
Baronesa de Maldá, 73
08950 Esplugues de Llobregat
Barcelona – SPAIN
Date of last revision of this leaflet:10/2017
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) http://www.aemps.es/.
- Country of registration
- Average pharmacy price3.14 EUR
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BECLO-RINO 50 micrograms Nasal Spray SuspensionDosage form: NASAL PRODUCT, 27.5 µgActive substance: fluticasone furoateManufacturer: Glaxosmithkline (Ireland) LimitedPrescription requiredDosage form: NASAL PRODUCT, 27.5 MICROGRAMS/SPRAYActive substance: fluticasone furoateManufacturer: Laboratorios Cinfa S.A.Prescription requiredDosage form: NASAL PRODUCT, 137 micrograms/50 micrograms/applicationActive substance: fluticasone, combinationsManufacturer: Laboratorios Cinfa S.A.Prescription required
Online doctors for BECLO-RINO 50 micrograms Nasal Spray Suspension
Discuss questions about BECLO-RINO 50 micrograms Nasal Spray Suspension, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











