
Beclonasal Aqua

Ask a doctor about a prescription for Beclonasal Aqua

How to use Beclonasal Aqua
Package Leaflet: Information for the User
Beclonasal Aqua, 50 micrograms/dose, nasal spray, suspension
beclometasone dipropionate
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, please inform your doctor or pharmacist. See section 4.
Table of Contents of the Leaflet:
- 1. What is Beclonasal Aqua and what is it used for
- 2. Important information before using Beclonasal Aqua
- 3. How to use Beclonasal Aqua
- 4. Possible side effects
- 5. How to store Beclonasal Aqua
- 6. Contents of the pack and other information
1. What is Beclonasal Aqua and what is it used for
The active substance of Beclonasal Aqua is beclometasone dipropionate. Beclometasone dipropionate belongs to a group of medicines called corticosteroids. When administered locally to the nasal mucosa, it reduces inflammation and swelling.
This medicine is used to prevent and treat seasonal and perennial allergic rhinitis, including hay fever, and vasomotor rhinitis (rhinitis caused by the dilation of blood vessels in the nasal mucosa).
2. Important information before using Beclonasal Aqua
When not to use Beclonasal Aqua:
- if you are allergic to beclometasone dipropionate or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting treatment with Beclonasal Aqua, discuss it with your doctor or pharmacist.
- if you have an infection or ulcer in the nose,
- if you have a tendency to recurrent nosebleeds,
- if you have nasal injuries or have undergone nasal surgery,
- if you have an untreated fungal, bacterial, or systemic viral infection,
- if you have asthma that is treated with steroids,
- if you have tuberculosis.
If you experience blurred vision or other vision disturbances, contact your doctor.
Children
Beclonasal Aqua is not recommended for use in children under 6 years of age due to insufficient clinical data.
Beclonasal Aqua and other medicines
Tell your doctor about all medicines you are taking now or have taken recently, as well as any medicines you plan to take, including those available without a prescription.
Concomitant use of Beclonasal Aqua and medicines containing corticosteroids, given orally or by inhalation, may cause adrenal suppression.
Some medicines may increase the effect of Beclonasal Aqua, so your doctor may recommend close monitoring if you are taking such medicines (including HIV infection medicines: ritonavir, cobicistat).
Pregnancy and breastfeeding
The use of this medicine in pregnant and breastfeeding women is decided by the doctor.
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor before using this medicine.
Driving and using machines
No effect on the ability to drive and use machines has been observed.
Beclonasal Aqua, nasal spray, contains benzalkonium chloride
This medicine contains 9 micrograms of benzalkonium chloride per single dose (0.09 ml), which corresponds to 0.1 mg of benzalkonium chloride per 1 ml of suspension. Benzalkonium chloride may cause irritation or swelling inside the nose, especially if used for a long time.
3. How to use Beclonasal Aqua
This medicine should always be used as directed by your doctor. The appropriate dosage and duration of treatment are determined by your doctor, depending on the type of disease. If you are unsure, consult your doctor or pharmacist.
The recommended dose for adults and children over 6 years of age is 1-2 sprays into each nostril twice a day. The total daily dose should not exceed eight single doses (four doses per nostril).
Beclonasal Aqua is not recommended for use in children under 6 years of age.
This medicine is used to prevent and treat allergic and vasomotor rhinitis. It should be used regularly. The medicine does not quickly reduce swelling of the nasal mucosa and discharge. The best therapeutic effect occurs after several days of treatment.
In the treatment of seasonal allergic rhinitis, administration of the medicine should be started before the onset of the pollen season.
The medicine does not treat allergic symptoms related to the eyes. Your doctor may recommend additional treatment for these symptoms.
Instructions for use
Before use:
- 1. Shake the bottle well and remove the protective cap.
- 2. Hold the bottle as shown in Figure 1; place the bottom of the bottle on the thumb and the actuator between the index and middle fingers.
- 3. Release the spray dose: To release the dose, press the actuator and the bottom of the bottle with your fingers. Before administering the first dose, perform three to six sprays into the air until a full dose is obtained. If the medicine has not been used for a longer period, make sure the released dose is full by pressing the actuator 1 or 2 times.
Using the medicine:
- 1. Blow your nose.
- 2. Shake the bottle well and remove the protective cap.
- 3. Hold the bottle as shown in Figure 1; place the bottom of the bottle on the thumb and the actuator between the index and middle fingers. To release the dose, press the actuator and the bottom of the bottle with your fingers.
- 4. Insert the actuator into the nostril as shown in Figure 2 and block the other nostril by gently pressing with your finger. Tilt your head slightly forward. Release one dose and at the same time take a calm and deep breath through your nose.
- 5. Remove the actuator from the nostril and breathe out through your mouth. If the medicine leaks
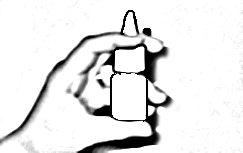
Figure 1.

Figure 2.
from the nostril, breathe in gently through your nose to draw in the medicine.
- 6. Repeat the actions described in points 4 and 5 to administer the medicine to the other nostril.
- 7. If you are using two doses in one nostril, repeat the actions described in points 4, 5, and 6.
- 8. After use, wipe the actuator and replace the protective cap.
Cleaning:
- 1. Detach the actuator from the bottle by gently pulling (Figure 3).
- 2. Rinse the actuator and protective cap with warm water.
- 3. Dry thoroughly.
- 4. Reassemble the parts.
Using a higher dose of Beclonasal Aqua than recommended
It is important to use the doses of the medicine as instructed on the packaging or as recommended by your doctor. Use only the dose recommended by your doctor. Using a higher or lower dose of the medicine may cause worsening of symptoms.
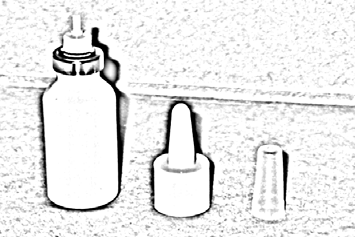
Figure 3.
Using high doses of beclometasone dipropionate for a long time may cause adrenal suppression, which can reduce the production of cortisol in the body.
Use the medicine as directed. If you (or someone else) use a higher dose of the medicine than recommended, consult your doctor immediately.
Missing a dose of Beclonasal Aqua
If you miss a dose of Beclonasal Aqua, take the next dose as scheduled. Do not take a double dose to make up for the missed dose.
Make sure you have enough medicine before going on holiday or traveling.
If you have any further questions about using this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
After using the medicine, you may experience mild sneezing and an unpleasant taste or smell.
Rarely (may affect up to 1 in 1000 people), the medicine may cause dryness and irritation of the nasal mucosa and throat, ulcers of the nasal mucosa, and perforation of the nasal septum (the membrane separating the nostrils), nosebleeds, headache, increased intraocular pressure (glaucoma).
Very rarely (may affect up to 1 in 10,000 people), the medicine may cause cataracts, allergic reactions, including rash, urticaria, itching, or redness, and swelling of the face, lips, or throat, or shortness of breath and (or) wheezing.
Blurred vision has been reported with an unknown frequency (frequency cannot be estimated from the available data).
In very rare cases, treatment with nasal corticosteroids may affect the normal production of steroids in the body. This is more likely if high doses are used for a long time.
Tell your doctor if:
- you experience pain in the nose or throat or nosebleeds after using the medicine,
- you experience eye symptoms that are not typical of hay fever, especially pain and blurred vision.
Additional side effects in children
One of the rare symptoms may be slowed growth in children. In children who use this medicine for a long time, the doctor will regularly monitor their growth.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, please inform your doctor or pharmacist. Side effects can be reported directly to the Department of Medicinal Product Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181 C, 02-222 Warsaw,
Phone: +48 22 49 21 301, Fax: + 48 22 49 21 309,
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Beclonasal Aqua
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the carton and bottle label after "EXP". The expiry date refers to the last day of the month.
Unopened bottle: Store in a temperature below 30 °C. Do not store in the refrigerator or freeze.
Opened bottle: After first opening, the shelf life is 6 months. Store in a temperature below 25 °C. Do not store in the refrigerator or freeze.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Beclonasal Aqua contains
- The active substance is beclometasone dipropionate. Each spray dose contains 50 micrograms of beclometasone dipropionate.
- The other ingredients are: polysorbate 80, glucose, cellulose dispersible (Avicel RC 591), benzalkonium chloride, sodium hydroxide, 1M hydrochloric acid or 1M sodium hydroxide (to adjust pH), purified water.
What Beclonasal Aqua looks like and contents of the pack
White or almost white suspension. Spray: fine mist
Pack size: 10 ml bottle containing 70 doses or 80 doses, 30 ml bottle containing 200 doses.
Marketing authorization holder
Orion Corporation
Orionintie 1
02200 Espoo
Finland
Manufacturer
Orion Corporation Orion Pharma
Volttikatu 8
FI-70700 Kuopio
Finland
Orion Corporation Orion Pharma
Joensuunkatu 7
FI-24100 Salo
Finland
To obtain more detailed information on this medicine, please contact the local representative of the marketing authorization holder:
Orion Pharma Poland Sp. z o.o.
[email protected]
Date of last revision of the leaflet:29.08.2022
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterOrion Corporation Orion Corporation Orion Pharma
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Beclonasal AquaDosage form: Aerosol, 50 mcg/doseActive substance: mometasoneManufacturer: FARMEA US Pharmacia Sp. z o.o.Prescription not requiredDosage form: Aerosol, (137 mcg + 50 mcg)/doseActive substance: fluticasone, combinationsPrescription requiredDosage form: Aerosol, (137 mcg + 50 mcg)/ doseActive substance: fluticasone, combinationsPrescription required
Alternatives to Beclonasal Aqua in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Beclonasal Aqua in Ukraine
Alternative to Beclonasal Aqua in Spain
Online doctors for Beclonasal Aqua
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Beclonasal Aqua – subject to medical assessment and local rules.








