
AXHIDROX 2.2 mg/pulse CREAM

How to use AXHIDROX 2.2 mg/pulse CREAM
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Axhidrox 2.2 mg/pulse cream
Glycopyrronium
Read the entire package leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Axhidrox and what is it used for
- What you need to know before starting to use Axhidrox
- How to use Axhidrox
- Possible side effects
- Storage of Axhidrox
- Package Contents and Additional Information
1. What is Axhidrox and what is it used for
Axhidrox contains the active ingredient glycopyrronium and belongs to a group of medications that reduce sweating called antihidrotics.
This medication is used for the topical treatment of primary axillary hyperhidrosis in adults.
Primary axillary hyperhidrosis causes excessive sweating in both armpits without any apparent reason, such as sports, hard physical work, hot weather, certain diseases, or medications. A characteristic of primary axillary hyperhidrosis is also that it occurs normally during the day, but not during sleep.
The use of this medication in the armpits leads to a reduction in sweat production in the sweat glands.
2. What you need to know before starting to use Axhidrox
Do not use Axhidrox
- if you are allergic to glycopyrronium or any of the other components of this medication (listed in section 6)
- if you have an eye disease where the pressure in your eye is high (glaucoma)
- if you have or have had an acute hemorrhage due to a heart or blood circulation disorder
- if you have a chronic inflammatory disease of the large intestine (severe ulcerative colitis)
- if you have or have had a chronic inflammation of the large intestine complicated by a severe extension of the colon (toxic megacolon complication of ulcerative colitis)
- if you have or have had an intestinal obstruction due to paralysis of the intestinal muscles (paralytic ileus)
- if you suffer from an immune response disease that affects the muscles (myasthenia gravis) or the salivary or tear glands (Sjögren's syndrome)
Warnings and Precautions
Consult your doctor or pharmacist before starting to use this medication
- if you have or have had prostate or bladder problems, or problems urinating.
Stop using this medication and consult your doctor if you notice symptoms of urinary retention, such as urinating with a weak flow or in drops, increased need to urinate, feeling of a full or insufficiently emptied bladder
- if you have severe kidney problems, including dialysis-dependent kidney failure.
- if you have blood-brain barrier disorders, such as after a traumatic brain injury in the last year, chemotherapy, radiation therapy to the head, skull and brain surgery, or due to intravenous drug abuse
- if you have heart disease, heart failure, irregular heartbeat, or high blood pressure
- if you have inflamed or damaged skin in the armpits, as this may increase the risk of local side effects. Use this medication only after the skin condition has completely resolved or after the wound has healed
Do not apply the cream to any other part of the body except the armpits, and avoid any contact of the cream with the eyes, nose, or mouth, or with other people.
Apply this medication only with the pump dispenser, not with your fingers. If the cream gets into your eyes, it can cause pupil dilation and blurred vision. If the cream gets into your mouth or nose, it can reduce saliva or nasal secretions. If your eyes, nose, or mouth come into contact with the cream, these areas should be rinsed immediately with plenty of water to reduce the risk of local side effects.
Cover the treated armpits with clothing during sexual intercourse, as side effects cannot be ruled out if others come into contact with the cream.
If you notice dry mouth, carefully clean your teeth. Visit your dentist regularly, as it may increase the risk of tooth decay.
Children
Do not use this medication in children and adolescents under 18 years of age, as it has not been studied in this age group.
Other Medications and Axhidrox
Tell your doctor or pharmacist if you are using, have recently used, or may need to use any other medication.
Some medications may affect or be affected by this medication
These medications include:
- topiramate, used to treat epilepsy and migraines
- sedating antihistamines, used to treat allergies or sleep disorders
- tricyclic antidepressants, used to treat depression
- monoamine oxidase inhibitors, used to treat depression or Parkinson's disease
- neuroleptics or antipsychotics, used to treat mental illnesses or anxiety
- opioids, used to treat pain or cough.
Pregnancy and Breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
There are no data on the use of this medication in pregnant women, and it is unknown whether the active ingredient of this medication passes into breast milk. Your doctor will tell you if you can use this medication during pregnancy. If you are breastfeeding, you and your doctor must decide whether to stop breastfeeding or stop treatment with this medication, considering the benefit of breastfeeding for your child and the benefit of treatment for you. This is because your baby should not come into contact with the cream or the treated skin.
Driving and Using Machines
After administration of this medication, blurred vision, drowsiness, fatigue, and dizziness (see section 4, "Possible Side Effects") may occur. In particular, blurred vision may occur if this medication comes into contact with the eyes. Do not drive, use machines, or perform hazardous work or sports until these effects disappear.
Axhidrox Contains Benzyl Alcohol, Propylene Glycol, and Cetostearyl Alcohol
This medication contains 2.7 mg of benzyl alcohol per pump actuation. Benzyl alcohol may cause allergic reactions and moderate local irritation.
This medication contains 8.1 mg of propylene glycol per pump actuation.
This medication may cause local skin reactions (such as contact dermatitis) because it contains cetostearyl alcohol.
3. How to Use Axhidrox
Follow your doctor's instructions for using this medication exactly. If you are unsure, consult your doctor or pharmacist again.
Apply this medication only to the skin of the armpits and only with the pump dispenser, not with your fingers. See section 2, "Warnings and Precautions".
The recommended dose is two pump actuations per armpit.
During the first 4 weeks of treatment, apply this medication to each armpit evenly, once a day, preferably at night.
From the 5th week onwards, you can reduce the frequency of application to twice a week, depending on the reduction in sweat production.
Preparing the Pump Before First Use
To obtain the recommended dose, you must remove the air trapped in the pump, as follows:
- Remove the pump cap.
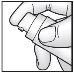 Place a piece of paper on the table. Hold the pump at an angle (see illustration), press the pump downwards repeatedly until the cream comes out of the opening.
Place a piece of paper on the table. Hold the pump at an angle (see illustration), press the pump downwards repeatedly until the cream comes out of the opening.- Slowly press the pump all the way down 10 more times and place the dispensed cream on the paper. Discard only the paper with the dispensed cream in the trash.
- The pump is now ready for use. Do notrepeat the pump preparation for subsequent uses.
Applying the Cream with the Pump Dispenser
- Remove the pump cap.
- Hold the pump in your hand with the pump opening facing the removed cap.
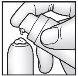 Press the pump all the way down twice to apply the recommended amount of cream to the top of the cap (see illustration).
Press the pump all the way down twice to apply the recommended amount of cream to the top of the cap (see illustration).- Distribute the cream evenly with the cap in one armpit.
- Repeat this process in the second armpit.
- Then, you must wash the pump cap, and for safety, also wash your hands completely and immediately with water and soap. This is important to avoid contact of the cream with the nose, eyes, or mouth. (See section 2, "Warnings and Precautions").
- Mark the number of treatments on the package table (see section 6). One treatment corresponds to 4 pump actuations, i.e., 2 pump actuations per armpit.
If You Use More Axhidrox Than You Should
It is unlikely that an overdose will occur if you use this medication only in the armpits as described.
However, if you apply this medication too frequently or in excess, the possible side effects (see section 4) may increase. Therefore, this medication should not be used on other parts (palms of the hands, feet, face) or on large areas of the body with increased sweating. Excessive sweating could cause overheating of the body and a possible heat stroke that can be life-threatening.
Stop using this medication and consult your doctor immediately if you notice an increase in the feeling of heat or body temperature.
If You Forget to Use Axhidrox
Do not use a double dose to make up for forgotten doses.
If You Stop Treatment with Axhidrox
If you or your doctor decide to stop using this medication, excessive sweating will reappear.
If you have any other questions about using this medication, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
Stop usingthis medication and contact your doctor or the nearest emergency service immediately if you experience any of the following serious side effects:
- swelling, mainly of the face, lips, or throat, which makes swallowing or breathing difficult, itching, and skin rash. This could be a sign of a severe allergic reaction or angioedema (frequency not known, cannot be estimated from available data), may require urgent medical treatment.
- Blurred vision (frequent side effect). (See section 2, "Driving and Using Machines").
The following additional side effects were observed
Very Common(may affect more than 1 in 10 people)
- dry mouth
Common(may affect up to 1 in 10 people)
- in the treated armpit: irritation, pain, itching, eczema, skin inflammation, skin rash, redness of the skin, nodules
- nasal dryness
- eye dryness
- skin dryness
- headache
- constipation
Uncommon(may affect up to 1 in 100 people)
- in the treated armpit: dryness, acne, lumps, swelling, skin hardening, scarring, small blisters, wound, pustules, inflamed hair follicles
- skin inflammation
- eczema
- itching, itching all over the body
- skin rash
- redness of the skin
- long-term skin eczema (atopic dermatitis)
- skin irritation
skin plaque (elevated, firm, and superficial skin changes larger than 1 cm in size)
- acne
- hives
- abnormal body odor
- skin condition similar to psoriasis (parapsoriasis)
- dryness of lips, hands, mucous membranes, throat
- lack of saliva
- stuffy nose
- itching, redness, or irritation of the eyes
- pupils of different sizes
- dilated pupils
- vision problems
- abdominal distension
- hard stools
- indigestion
- nausea
- pain in the mouth and throat
- throat tightness
- drowsiness
- fatigue
- attention disorders
- anxiety
- restlessness
- sleep disorders, poor sleep quality
- dizziness
- headaches
- urinary evacuation disorders
- excessive sweating
- reduced platelet count in blood observed in a blood test
- increased heart rate
- heart rhythm disorder (called "prolonged QT interval", observed in the ECG, heart electrical activity)
- increased liver enzymes, bilirubin, and red blood cell volume observed in a blood test
- decreased hemoglobin concentration in red blood cells observed in the blood test
Reporting Side Effects
If you experience any side effects, consult your doctor or pharmacist, even if they are possible side effects not listed in this package leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medications: www.notificaRAM.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Axhidrox
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date stated on the label and on the packaging after CAD:. The expiration date is the last day of the month indicated.
This medication does not require special storage conditions.
After the first pump actuation, the medication can be used for a maximum of 12 months.
Medications should not be disposed of through wastewater or household waste. Deposit the packaging and medications you no longer need at the Sigre Collection Point in the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Axhidrox Composition
- The active ingredient is glycopyrronium (as glycopyrronium bromide).
1 g of cream contains glycopyrronium bromide, equivalent to 8 mg of glycopyrronium. One pump actuation delivers 270 mg of cream, which contains glycopyrronium bromide, corresponding to 2.2 mg of glycopyrronium.
- The other ingredients are benzyl alcohol (E1519), propylene glycol (E1520), and cetostearyl alcohol (see section 2), citric acid (E330), glycerol monostearate 40-55, glycerol monostearate macrogol 20, sodium citrate (E331), octyldodecanol, and purified water.
Product Appearance and Package Contents
Axhidrox is a white, glossy cream, available in a multidose container with a pump and cap. The multidose container contains 50 g of cream. After preparing the pump, it will provide 124 pump actuations, which are sufficient for 31 treatments for both armpits.
Mark the number of treatments on the package table (see section 6). After 31 treatments, do not continue using the pump, even if it is not completely empty.
Marketing Authorization Holder
Industrial Farmacéutica Cantabria, S.A.
Barrio Solía 30
La Concha de Villaescusa
39690 Cantabria (Spain)
Manufacturer
Dr. August Wolff GmbH & Co. KG Arzneimittel
Sudbrackstrasse 56
33611 Bielefeld
Germany
Date of Last Revision of this Package Leaflet:February 2024
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AXHIDROX 2.2 mg/pulse CREAMDosage form: INJECTABLE, 1 mlActive substance: tralokinumabManufacturer: Leo Pharma A/SPrescription requiredDosage form: INJECTABLE, 300 mgActive substance: tralokinumabManufacturer: Leo Pharma A/SPrescription requiredDosage form: CAPSULE, 10 mgActive substance: alitretinoinManufacturer: Industrial Farmaceutica Cantabria S.A.Prescription required
Online doctors for AXHIDROX 2.2 mg/pulse CREAM
Discuss questions about AXHIDROX 2.2 mg/pulse CREAM, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions








