ADTRALZA 300 mg Injectable Solution in a Pre-filled Pen

How to use ADTRALZA 300 mg Injectable Solution in a Pre-filled Pen
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Adtralza 300 mg solution for injection in pre-filled pen
tralokinumab
This medicinal product is subject to additional monitoring, which will allow for the quick identification of new safety information. You can help by reporting any side effects you may have. The last section of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Adtralza and what is it used for
- What you need to know before you use Adtralza
- How to use Adtralza
- Possible side effects
- Storage of Adtralza
- Contents of the pack and further information
1. What is Adtralza and what is it used for
Adtralza contains the active substance tralokinumab.
Tralokinumab is a monoclonal antibody (a type of protein) that blocks the action of a protein called IL-13. IL-13 plays a key role in the occurrence of symptoms of atopic dermatitis.
Adtralza is used to treat adult and adolescent patients from 12 years of age with moderate to severe atopic dermatitis, also known as atopic eczema. Adtralza can be used alone or in combination with other medicines for atopic eczema that are applied to the skin.
The use of Adtralza to treat atopic dermatitis may improve your eczema and reduce the itching and skin pain associated with it.
2. What you need to know before you use Adtralza
Do not use Adtralza
- if you are allergic to tralokinumab or any of the other ingredients of this medicine (listed in section 6).
If you think you may be allergic, or are unsure, consult your doctor, pharmacist, or nurse before using Adtralza.
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting treatment with Adtralza.
Allergic reactions
Very rarely, medicines may cause allergic reactions (hypersensitivity) and severe allergic reactions called anaphylactic reactions. While using Adtralza, you should watch for signs of these reactions (i.e., breathing problems, swelling of the face, mouth, and tongue, fainting, dizziness, feeling of dizziness (due to low blood pressure), hives, itching, and skin rash). Stop using Adtralza and contact your doctor or seek immediate medical attention if you notice any sign of an allergic reaction. Such signs are indicated at the beginning of section 4.
Intestinal parasitic infection
Adtralza may reduce your resistance to infections caused by parasites. Any parasitic infection should be treated before starting treatment with Adtralza. Consult your doctor if you have diarrhea, gas, stomach discomfort, greasy stools, and dehydration, which could be signs of parasitic infection. If you live in a region where these infections are common or if you travel to that region, consult your doctor.
Eye problems
Consult your doctor if you have new or worsening eye problems, including eye pain or changes in vision.
Children
The safety and benefits of Adtralza in children under 12 years of age are still unknown, so do not administer this medicine to this population.
Other medicines and Adtralza
Tell your doctor or pharmacist
- if you are using, have recently used, or might use any other medicines.
- if you have recently been given or are to be given any vaccine.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine. The effects of Adtralza in pregnant women are not known; therefore, it is preferable to avoid its use during pregnancy unless your doctor advises you to use it.
If you proceed, you and your doctor will need to decide whether to breastfeed or use Adtralza. You should not do both at the same time.
Driving and using machines
It is unlikely that Adtralza will affect your ability to drive or use machines.
Adtralza contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per 300 mg dose; this is essentially "sodium-free".
3. How to use Adtralza
Follow exactly the administration instructions of this medicine given by your doctor, pharmacist, or nurse. In case of doubt, consult your doctor, pharmacist, or nurse again.
Each pre-filled pen contains 300 mg of tralokinumab.
Amount of Adtralza to be administered and duration of treatment
- Your doctor will decide the amount of Adtralza you need and the duration of treatment.
- The recommended first dose is 600 mg (two 300 mg injections), followed by 300 mg (one 300 mg injection) administered every 2 weeks. Depending on how you respond to treatment, your doctor will decide if you can be administered a dose every 4 weeks.
Adtralza is administered by injection under your skin (subcutaneous injection). Your doctor or nurse and you can decide if you can inject Adtralza yourself.
Inject Adtralza only after your doctor or nurse has taught you how to do it correctly. A caregiver can also inject Adtralza after receiving proper training.
Do not shake the pen.
Read the "Instructions for use" carefully before injecting Adtralza.
If you use more Adtralza than you should
If you use more medicine than you should or if you have been given the dose too soon, consult your doctor, pharmacist, or nurse.
If you forget to use Adtralza
If you have forgotten to inject a dose at the right time, inject Adtralza as soon as possible.
Subsequently, the next dose should be injected according to the established schedule.
If you stop treatment with Adtralza
Do not stop treatment with Adtralza without consulting your doctor first.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Adtralza can cause serious side effects, including allergic reactions (hypersensitivity) such as anaphylactic reactions; the signs may include:
- breathing problems
- swelling of the face, mouth, and tongue
- fainting, dizziness, feeling of dizziness (low blood pressure)
- hives
- itching
- skin rash
Stop using Adtralza and contact your doctor or seek immediate medical attention if you notice any sign of an allergic reaction.
Other side effects
Very common(may affect more than 1 in 10 people)
- upper respiratory tract infections (i.e., common cold and sore throat)
Common(may affect up to 1 in 10 people)
- redness and itching of the eyes
- eye infection
- reactions at the injection site (i.e., redness, swelling)
Uncommon(may affect up to 1 in 100 people)
- eye inflammation that can cause eye pain or decreased vision
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Adtralza
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and on the carton after EXP. The expiry date is the last day of the month stated.
Store in the original package to protect from light.
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
If necessary, Adtralza can be stored at room temperature up to 30°C in the original package for a maximum of 14 days. Do not store above 30°C. Discard Adtralza if not used within 14 days of storage at room temperature.
If you need to remove the package from the refrigerator permanently, write the date you removed it on the package and use Adtralza within 14 days. Adtralza should not be refrigerated again during this period.
Do not use this medicine if you notice it is cloudy, discolored, or contains particles.
Medicines should not be disposed of via wastewater or household waste. Ask your doctor, pharmacist, or nurse how to dispose of the package and any unused medicine. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Adtralza
- The active ingredient is tralokinumab.
- Each pre-filled pen contains 300 mg of tralokinumab in 2 ml of injectable solution.
- The other components are sodium acetate trihydrate (E262), acetic acid (E260), sodium chloride, polysorbate 80 (E433), and water for injectable preparations.
Appearance of Adtralza and Container Contents
Adtralza is a transparent to opalescent, colorless to pale yellow solution, supplied in a pre-filled pen.
Adtralza is available in single-unit containers containing 2 pre-filled pens or in multi-unit containers containing 6 (3 units of 2) pre-filled pens.
Only certain pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
LEO Pharma A/S
Industriparken 55
DK-2750 Ballerup
Denmark
You can request more information about this medicinal product from the local representative of the marketing authorization holder:
België/Belgique/Belgien LEO Pharma N.V./S.A Tél/Tel: +32 3 740 7868 | Lietuva LEO Pharma A/S Tel: +45 44 94 58 88 |
LEO Pharma A/S Teπ.: +45 44 94 58 88 | Luxembourg/Luxemburg LEO Pharma N.V./S.A Tél/Tel: +32 3 740 7868 |
Ceská republika LEO Pharma s.r.o. Tel: +420 734 575 982 | Magyarország LEO Pharma A/S Tel: +45 44 94 58 88 |
Danmark LEO Pharma AB Tlf: +45 70 22 49 11 | Malta LEO Pharma A/S Tel: +45 44 94 58 88 |
Deutschland LEO Pharma GmbH Tel: +49 6102 2010 | Nederland LEO Pharma B.V. Tel: +31 205104141 |
Eesti LEO Pharma A/S Tel: +45 44 94 58 88 | Norge LEO Pharma AS Tlf: +47 22514900 |
Ελλάδα LEO Pharmaceutical Hellas S.A. Τηλ: +30 210 68 34322 | Österreich LEO Pharma GmbH Tel: +43 1 503 6979 |
España Laboratorios LEO Pharma, S.A. Tel: +34 93 221 3366 | Polska LEO Pharma Sp. z o.o. Tel.: +48 22 244 18 40 |
France Laboratoires LEO Tél: +33 1 3014 4000 | Portugal LEO Farmacêuticos Lda. Tel: +351 21 711 0760 |
Hrvatska LEO Pharma A/S Tel: +45 44 94 58 88 | Ireland LEO Laboratories Ltd Tel: +353 (0) 1 490 8924 |
România LEO Pharma A/S Tel: +45 44 94 58 88 | Slovenija LEO Pharma A/S Tel: +45 44 94 58 88 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika LEO Pharma s.r.o. Tel: +420 734 575 982 |
Italia LEO Pharma S.p.A Tel: +39 06 52625500 | Suomi/Finland LEO Pharma Oy Puh/Tel: +358 20 721 8440 |
Κύπρος The Star Medicines Importers Co. Ltd. Τηλ: +357 2537 1056 | Sverige LEO Pharma AB Tel: +46 40 3522 00 |
Latvija LEO Pharma A/S Tel: +45 44 94 58 88 | United Kingdom (Northern Ireland) LEO Laboratories Ltd Tel: +44 (0) 1844 347333 |
Date of Last Revision of this Leaflet:
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
Instructions for use with information on how to inject Adtralza are available on the other side of this leaflet.
Instructions for Use:
Adtralza 300 mg Solution for Injection in Pre-filled Pen
tralokinumab
Read these instructions before starting to use the Adtralza pre-filled pens and each time you get a new pack, as they may have been updated. You can also talk to your healthcare professional about your condition or treatment.
Keep these instructions for use to be able to refer to them again if needed.
Each pre-filled pen contains 300 mg of tralokinumab.
The Adtralza pre-filled pens are for single use only.
IMPORTANT INFORMATION
Important information you need to know before injecting Adtralza
- Before injecting Adtralza for the first time, your healthcare professional will show you how to prepare and inject Adtralza using the pre-filled pen.
- Do notinject Adtralza until you have been shown how to inject it correctly.
- Talk to your healthcare professional if you have any questions about how to inject Adtralza correctly.
- To receive the full dose, you must administer 1 injection of Adtralza.
- It is recommended that you use a different injection site for each new injection.
- The Adtralza pre-filled pen has a needle shield that will automatically cover the needle after the injection is complete.
- Do notremove the cap until just before administering the injection.
- Do notshare or reuse the Adtralza pre-filled pens.
Parts of the Adtralza Pre-filled Pen:
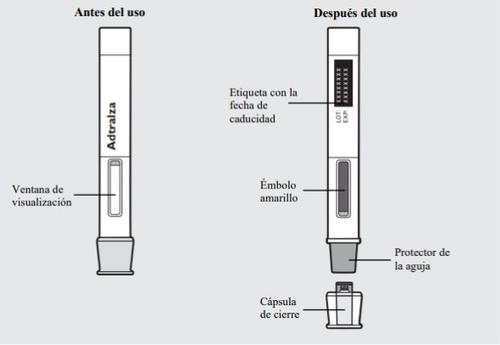
Storage of Adtralza
- Keep Adtralza and all medicines out of the sight and reach of children.
- Store the Adtralza pre-filled pens in the refrigerator between 2 °C and 8 °C.
- Keep the Adtralza pre-filled pens in the original package to protect them from light until you are ready to use them.
- Do notfreeze the Adtralza pre-filled pens. Do notuse them if they have been frozen.
- Adtralza can be stored in the original package at room temperature up to 30 °C for a maximum of 14 days. If you need to take the package out of the refrigerator permanently, write the date you took it out on the package and use Adtralza within 14 days. Discard the pens if they have been out of the refrigerator for more than 14 days.
Step 1: Preparation of the Adtralza Injection
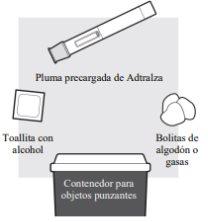
1a: Gather all the necessary materials for the injection
For each dose of Adtralza, you will need:
- A flat, clean, and well-lit work surface, such as a table
- 1 Adtralza pre-filled pen
- An alcohol swab (not included in the package)
- Clean cotton balls or gauze (not included in the package)
- A sharps container for disposal of needles (not included in the package).
1b: Take the Adtralza package out of the refrigerator
- Check the expiration date (EXP) on the package. Do notuse the pre-filled pen if the expiration date has passed.
- When using the first pre-filled pen from the package, check that the package seal is intact. Do notuse the Adtralza pre-filled pens if the package seal is broken.
Do not usethe Adtralza pre-filled pens that have been stored at room temperature for more than 14 days.
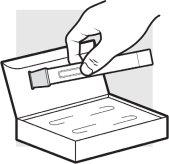
1c: Remove the Adtralza pre-filled pen from the package
Remove 1pre-filled pen from the package. When using the first pre-filled pen, return the package with the remaining pre-filled pen to the refrigerator.
- Do notremove the cap from the pre-filled pen until you reach step 3 and you are ready for the injection.
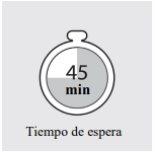
1d: Allow the Adtralza pre-filled pen to reach room temperature
Place the pre-filled pen on a flat surface and wait at least 45 minutes before injecting Adtralza, to allow the pre-filled pen to reach room temperature (between 20 °C and 30 °C).
This will help make the Adtralza injection more comfortable.
- Do notheat the pre-filled pen in any way.
- Do notshake the pre-filled pen.
- Do notreturn the pre-filled pen to the refrigerator once it has reached room temperature.
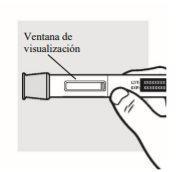
1e: Check the Adtralza pre-filled pen
- Make sure the label shows the correct name of the medicine, Adtralza.
- Check the expiration date on the label of the pre-filled pen.
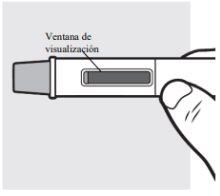
- Look at the medicine through the viewing window. The medicine should be transparent to opalescent, colorless to pale yellow.
- You may see small air bubbles in the liquid. This is normal; you do not need to do anything about it.
- Do not use the Adtralza pre-filled pen if:
- the expiration date on the pre-filled pen has passed
- the medicine looks cloudy, discolored, or contains particles
- the pre-filled pen looks damaged or has been dropped
If you cannot use the pre-filled pen, discard it in a sharps container and use a new pre-filled pen.
Step 2: Choosing and Preparing the Injection Site
| |
Injection administered only by the caregiver | |
Injection administered by oneself or by the caregiver |
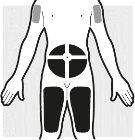
2a: Choose the area where you will administer the injection
- You can inject into:
- the stomach area (abdomen)
- the thigh
- the upper arm, only when the caregiver administers the injection.
- Do notinject the medicine into sensitive skin, skin with bruises, scaly skin, skin with scars, hardened skin, or skin with eczema.
- Do notadminister the injection within 5 cm around the navel.
- It is recommended that you use a different injection site for each new injection. Do not use the same area of the body 2 times in a row.
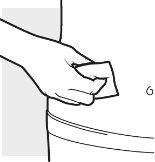
2b: Wash your hands and prepare the skin
- Wash your hands with soap and water.
- Clean the chosen area with an alcohol swab, using a circular motion.
- Let the area dry completely.
- Do notblow on or touch the cleaned area before the injection.
Step 3: Injecting Adtralza
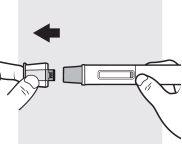
3a: Remove the cap from the Adtralza pre-filled pen
Hold the pre-filled pen with one hand, and with the other, pull the cap straight off and throw it away in a sharps container. At this point, the needle shield is exposed to prevent you from touching the needle.
- Do not try to put the cap back on.This could cause the injection to happen too soon or the needle to become damaged.
- Do nottry to touch or press the needle shield with your finger. This could cause a needlestick injury.
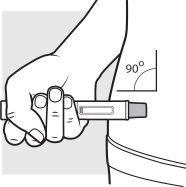
3b: Place the Adtralza pre-filled pen on the injection site so you can see the viewing window
You can gently pinch the cleaned skin or administer the injection without pinching the skin.
Follow the instructions for healthcare professionals on how to administer the injection,
- Place the needle shield of the pre-filled pen against the skin at a 90-degree angle in the injection site you cleaned earlier. Make sure you can see the viewing window.
- Do notchange the position of the pre-filled pen after the injection has started.
If the pre-filled pen is removed too soon, you may see some medicine leaking from the pre-filled pen. If this happens, you may not have received the full dose. Call your doctor, pharmacist, or nurse.
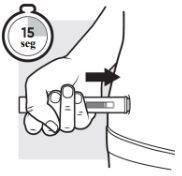
3c: Press the Adtralza pre-filled pen and hold it in place
Press the pre-filled pen firmly and hold it in place. You will hear a “click”that tells you the injection has started, and the yellow plunger will begin to move.
The yellow plunger will move down to the bottom of the viewing window as the medicine is injected.
Injecting the full dose may take up to 15 seconds.
You will hear a second “click”when the yellow plunger fills the viewing window.
Continue to press.
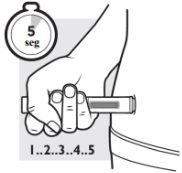
3d: Continue to press for about 5 seconds more
After you hear the second “click”, continue to press the pre-filled pen firmly against your skin for 5 seconds to make sure you receive the full dose.
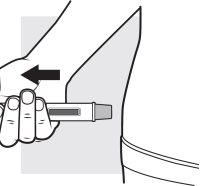
3e: Remove the Adtralza pre-filled pen
Remove the pre-filled pen from the injection site. The needle shield will slide and lock over the needle.
- Place a cotton ball or a dry gauze over the injection site for a few seconds. Do notrub the injection site.
- You may see a small amount of blood or liquid at the injection site. This is normal. If necessary, cover the injection site with a small bandage.
| Before use: |
| |
After use: | |
|
3f: Check the viewing window
Check the viewing window to make sure all the liquid has been injected. If the yellow plunger does not cover the viewing window, you may not have received the full dose. If this happens or if you have any other questions, call your doctor, pharmacist, or nurse.
Step 4: Disposal of the Adtralza Pre-filled Pen
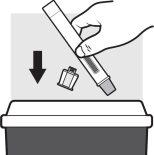
- Discard the used Adtralza pre-filled pen in a sharps container immediately after use.
- Do notthrow the Adtralza pre-filled pen away with household waste.
- If you do not have a sharps container, you can make one at home that:
- is made of sturdy plastic;
- can be closed with a tight-fitting, puncture-resistant lid, so that sharp objects cannot stick out,
- is upright and stable during use,
- is leak-proof and
- is properly labeled to warn of the hazardous waste it contains.
- When the puncture-resistant container is almost full, you should follow your local guidelines for the disposal of sharps containers.
- Do notrecycle the puncture-resistant container.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ADTRALZA 300 mg Injectable Solution in a Pre-filled PenDosage form: INJECTABLE, 1 mlActive substance: tralokinumabManufacturer: Leo Pharma A/SPrescription requiredDosage form: CAPSULE, 10 mgActive substance: alitretinoinManufacturer: Industrial Farmaceutica Cantabria S.A.Prescription requiredDosage form: CAPSULE, 30 mgActive substance: alitretinoinManufacturer: Industrial Farmaceutica Cantabria S.A.Prescription required
Online doctors for ADTRALZA 300 mg Injectable Solution in a Pre-filled Pen
Discuss questions about ADTRALZA 300 mg Injectable Solution in a Pre-filled Pen, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions