
How to use Axhidrox
Package Leaflet: Information for the User
Axhidrox
2.2 mg/dose, cream
Glycopyrronium
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- 1. What is Axhidrox and what is it used for
- 2. Important information before using Axhidrox
- 3. How to use Axhidrox
- 4. Possible side effects
- 5. How to store Axhidrox
- 6. Contents of the pack and other information
1. What is Axhidrox and what is it used for
Axhidrox contains the active substance glycopyrronium and belongs to a group of medicines that reduce sweat production, called antihidrotics.
Axhidrox is used for the local treatment of severe primary axillary hyperhidrosis in adults.
Primary axillary hyperhidrosis causes excessive sweating in both armpits without any obvious reason, such as sports, heavy physical work, hot weather, certain diseases, or medicines. A characteristic feature of primary axillary hyperhidrosis is that it usually occurs during the day, but not during sleep.
Applying Axhidrox externally to the armpits leads to a reduction in sweat production in the sweat glands.
2. Important information before using Axhidrox
When not to use Axhidrox
Warnings and precautions
Before starting treatment with Axhidrox, discuss it with your doctor or pharmacist if you have or have had:
- problems with the prostate or urinary bladder or problems with urination. Stop using the medicine and consult a doctor if you experience symptoms of urinary retention, such as urinating with a weak stream or drops, increased need to urinate, or feeling of a full or insufficiently emptied bladder;
- severe kidney problems, including kidney failure requiring dialysis;
- blood-brain barrier disorders, such as after traumatic brain injury within the last year, chemotherapy, radiation therapy in the head, skull or brain surgery, or due to intravenous drug abuse;
- heart disease, heart failure, irregular heartbeat, or high blood pressure;
- skin inflammation or injury in the armpit area, as this may increase the risk of local side effects. Axhidrox should only be used after the skin condition or wound has completely healed.
Do not apply the cream to other parts of the body than the armpits and avoid contact with the eyes, nose, mouth, or other people with the cream.
- Axhidrox should only be applied using the pump cap, not with fingers. If the cream gets into the eyes, it may cause temporary pupil dilation and blurred vision. If the cream gets into the mouth or nose, it may cause reduced saliva or nasal secretion. In case of contact with the eyes, nose, or mouth, rinse these areas immediately with plenty of water to reduce the risk of local side effects.
- During sexual intercourse, cover the treated armpits with clothing, as it cannot be excluded that side effects may occur in case of contact with the cream by other people.
If you experience dry mouth, brush your teeth thoroughly. Regularly check your teeth with a dentist, as the risk of tooth decay may be higher.
Children and adolescents
Axhidrox must not be used in children and adolescents under 18 years of age, as the safety and efficacy of this medicine have not been established in this age group.
Axhidrox and other medicines
Tell your doctor or pharmacist about all medicines you are taking or have recently taken, as well as any medicines you plan to take.
Some medicines may affect Axhidrox or Axhidrox may affect them.
These medicines include:
- topiramate, used to treat epilepsy and migraines
- sedating antihistamine medicines, used to treat allergies or sleep disorders
- tricyclic antidepressants, used to treat depression
- monoamine oxidase inhibitors, used to treat depression or Parkinson's disease
- neuroleptics or antipsychotic medicines, used to treat mental illness or anxiety
- opioids, used to treat pain or cough.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
There is no data on the use of Axhidrox in pregnant women, and it is not known whether the active substance of this medicine passes into human milk. Your doctor will discuss with you whether you can use Axhidrox during pregnancy. If you are breastfeeding, a decision must be made with your doctor whether to discontinue breastfeeding or discontinue Axhidrox treatment, considering the benefit of breastfeeding to the baby and the benefit of treatment to the mother. This is because the baby should not come into contact with the cream or the treated skin.
Driving and using machines
After using Axhidrox, blurred vision, drowsiness, fatigue, and dizziness may occur (see section 4).
Blurred vision may occur especially if Axhidrox gets into the eyes. Do not drive, operate machinery, or perform hazardous work or sports until these symptoms have resolved.
Axhidrox contains benzyl alcohol, propylene glycol, and cetearyl alcohol
The medicine contains 2.7 mg of benzyl alcohol in each dose of the medicine released by pressing the pump. Benzyl alcohol may cause allergic reactions and mild local irritation.
The medicine contains 8.1 mg of propylene glycol in each dose of the medicine released by pressing the pump.
The medicine contains cetearyl alcohol, which may cause a local skin reaction (e.g., contact dermatitis).
3. How to use Axhidrox
This medicine should always be used exactly as your doctor has told you. If you are not sure, ask your doctor or pharmacist.
Axhidrox should only be applied to the armpit skin and only using the pump cap, not with fingers (see section 2, "Warnings and precautions").
The recommended dose is two pump actuations per armpit.
During the first 4 weeks of treatment, Axhidrox should be applied evenly to each armpit, once a day, preferably in the evening.
From the 5th week onwards, the frequency of application can be reduced to 2 times a week, depending on the degree of sweat reduction.
Preparing the pump before first use
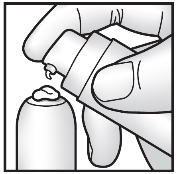
To obtain the recommended dose, remove the air from the pump as follows:
- Remove the cap from the pump.
- Place a piece of paper on the table. Hold the pump at an angle (see diagram) and press the pump several times until the cream comes out of the opening.
- Press the pump slowly to the end, 10 more times, and apply the expressed cream to the piece of paper. Dispose of the paper with the expressed cream in the trash only.
- The pump is now ready for use. It is not necessary to prepare the pump again before the next use.
Applying the cream with the pump cap
- Remove the cap from the pump.
- Hold the pump in one hand, with the pump opening facing the removed cap.
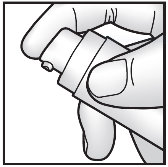
- Press the pump twice to the end to apply the recommended amount of cream to the top of the cap (see diagram).
- Use the cap to spread the cream evenly under one armpit.
- Repeat this process for the other armpit.
- Then, for safety, immediately and thoroughly wash the cap and hands with soap and water. It is important to avoid contact with the cream, nose, eyes, or mouth (see section 2, "Warnings and precautions").
- Mark the next dose number in the table on the carton (see section 6). One dose corresponds to 4 pump actuations, i.e., 2 pump actuations per armpit.
Using a higher dose of Axhidrox than recommended
Overdose is unlikely if Axhidrox is used only under the armpits as described.
However, if Axhidrox is used too frequently or in excessive amounts, possible side effects may worsen (see section 4).
Therefore, Axhidrox should not be used on other parts of the body (hands, feet, face) or large areas of the body with increased sweating. Excessive sweating restriction may lead to overheating and possible life-threatening heat stroke. Stop using Axhidrox and consult a doctor immediately if you experience worsening feelings of heat or elevated body temperature.
Missing a dose of Axhidrox
Do not take a double dose to make up for a forgotten dose.
Stopping Axhidrox treatment
If you or your doctor decide to stop using Axhidrox, excessive sweating will occur again.
If you have any further questions about using this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Axhidrox can cause side effects, although not everybody gets them.
Stop usingAxhidrox and contact your doctor or the emergency department of your nearest hospital if you experience the following serious side effects:
- swelling, mainly of the face, lips, or throat, which causes difficulty swallowing or breathing, itching, and rash. This may be a sign of a severe allergic reaction or angioedema (frequency not known, cannot be estimated from the available data), and you may need urgent medical attention.
- blurred vision (common side effects) (see section 2, "Driving and using machines").
Other observed side effects:
Very commonside effects (may affect more than 1 in 10 people)
- dry mouth.
Commonside effects (may affect up to 1 in 10 people)
- in the treated armpit: irritation, pain, itching, rash, skin inflammation, redness of the skin, bumps
- dry nose
- dry eyes
- dry skin
- headache
- constipation.
Uncommonside effects (may affect up to 1 in 100 people)
- in the treated armpit: dryness, acne, swelling, skin hardening, scar, small blisters, wound, pimples, folliculitis
- rash
- itching, itching all over the body
- skin rash
- redness of the skin
- long-lasting skin rash (atopic dermatitis)
- skin irritation
- skin plaques (elevated, hard, superficial skin changes larger than 1 cm)
- acne
- hives
- unusual body odor
- skin disease resembling psoriasis (psoriasiform dermatitis)
- dry lips, hands, mucous membranes, throat
- lack of saliva
- feeling of a blocked nose
- itchy, red, or irritated eyes
- different pupil sizes
- dilated pupils
- impaired vision
- abdominal bloating
- hard stools
- indigestion
- nausea
- mouth and throat pain
- feeling of throat constriction
- drowsiness
- fatigue
- attention disorders
- anxiety
- psychomotor agitation
- sleep disorders, poor sleep quality
- dizziness
- feeling of discomfort in the head
- urination disorders
- excessive sweating
- reduced platelet count, observed in blood tests
- rapid heartbeat
- change in heart rhythm (so-called "QT interval prolongation" visible in ECG, electrical activity of the heart)
- increased liver enzyme activity, bilirubin levels, and red blood cell volume observed in blood tests
- reduced hemoglobin levels in red blood cells observed in blood tests.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Axhidrox
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after "EXP". The expiry date refers to the last day of that month.
There are no special storage instructions for this medicine.
Shelf life after first opening of the container: 12 months.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
What Axhidrox contains
- The active substance is glycopyrronium (as glycopyrronium bromide). 1 g of cream contains glycopyrronium bromide equivalent to 8 mg of glycopyrronium. One dose (one pump actuation) delivers 270 mg of cream containing glycopyrronium bromide equivalent to 2.2 mg of glycopyrronium.
- The other ingredients are: benzyl alcohol (E 1519), propylene glycol (E 1520), and cetearyl alcohol (see section 2), citric acid (E 330), glycerol monostearate 40-55, macrogol-20-glycerol monostearate, sodium citrate (E 331), octyldodecanol, and purified water.
What Axhidrox looks like and contents of the pack
Axhidrox is a white, shiny cream, available in packs containing one multidose container with a pump and cap. The multidose container contains 50 g of cream. After preparation, 124 pump actuations can be performed, sufficient for 31 doses for both armpits.
After each dose, mark the next dose number in the table on the carton. After 31 doses, do not use the pump, even if the multidose container is not completely empty.
Marketing authorization holder and manufacturer
Dr. August Wolff GmbH & Co. KG Arzneimittel
Sudbrackstrasse 56
33611 Bielefeld, GERMANY
Phone: +49 521 8808-05;
Fax: +49 521 8808-334
Email: [email protected]
To obtain more detailed information on this medicine, contact the local representative of the marketing authorization holder.
SOLPHARM Sp. z o.o.
ul. Zakątek 1
05-270 Marki
Phone: +48 /22/ 616 28 08
Email: [email protected]
This medicine is authorized in the Member States of the European Economic Area under the following names:
Austria
Axhidrox 2,2 mg/Pumpenhub Creme
Belgium
Axhidroks 8 mg/g crème
Bulgaria
АКСХИДРОКС 2,2 mg/изпомпване, крем
Croatia
Axhidrox 2,2 mg po potisku krema
Czech Republic
Axhidrox
Denmark
Axhidrox
Estonia
Axhidrox 8 mg/g kreem
Finland
Axhidrox 2,2 mg/pumpun käyttökerta emulsiovoide
France
GLYCOPYRRONIUM WOLFF 8 mg/g, crème
Greece
AXHIDROX
Netherlands
Axhidrox 8 mg/g, Crème
Ireland
Axhidrox 2.2 mg/pump actuation cream
Latvia
Axhidrox 2,2 mg/dozējumā krēms
Lithuania
Akshidroz 8 mg/g kremas
Luxembourg
Axhidroks 8 mg/g crème
Germany
Axhidrox 2,2 mg/Hub Creme
Norway
Axhidrox 2,2 mg/pumpetrykk krem
Poland
Axhidrox
Romania
Axhidrox 2,2 mg/doza, cremă
Slovakia
Axhidrox
Slovenia
Axhidrox 2,2 mg/potisk krema
Sweden
Axhidrox
Hungary
Axhidrox 8 mg/g krém
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterDr. August Wolff GmbH & Co. Arzneimittel
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AxhidroxDosage form: Solution, 20 mg/mlActive substance: minoxidilManufacturer: Industrial Farmaceutica Cantabria, S.A.Prescription not requiredDosage form: Solution, 50 mg/mlActive substance: minoxidilManufacturer: Industrial Farmaceutica Cantabria, S.A.Prescription not requiredDosage form: Aerosol, 50 mg/mlActive substance: minoxidilManufacturer: mibe GmbH Arzneimittel Sun-Farm Sp. z o.o.Prescription not required
Alternatives to Axhidrox in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Axhidrox in España
Alternative to Axhidrox in Ucrania
Online doctors for Axhidrox
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Axhidrox – subject to medical assessment and local rules.







