
Furocort
Ask a doctor about a prescription for Furocort

How to use Furocort
Leaflet accompanying the packaging: information for the user
Furocort, 27.5 micrograms/dose, nasal spray, suspension
Fluticasone furoate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, please tell your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Furocort and what is it used for
- 2. Important information before using Furocort
- 3. How to use Furocort
- 4. Possible side effects
- 5. How to store Furocort
- 6. Contents of the packaging and other information Detailed instructions for using the nasal spray
1. What is Furocort and what is it used for
Furocort (fluticasone furoate) belongs to a group of medicines called glucocorticosteroids.
Furocort reduces inflammation caused by allergies (rhinitis) and thus
relieves allergy symptoms.
Furocort, nasal spray is used to treat symptoms of allergic rhinitis, including stuffy nose, runny nose, and itching of the nose, sneezing, tearing, itching or redness of the eyes in adults, adolescents, and children aged 6 and older.
Allergy symptoms may occur at a certain time of the year and may be caused by an allergy to grass or tree pollen (hay fever), or may occur all year round and are most often caused by an allergy to animals, house dust mites, or fungi.
2. Important information before using Furocort
When not to use Furocort
- If you are allergic to fluticasone furoate or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Children and adolescents
Do not use in children under 6 years of age.
Using Furocort:
- may cause growth retardation in children if used for a long time. Your doctor will regularly check your child's growth and make sure they are using the lowest effective dose.
- may cause eye diseases, such as glaucoma (increased pressure in the eye) or cataracts (clouding of the lens in the eye). Tell your doctor if you have had such changes in the past or if you experience blurred vision or other vision disturbances while using Furocort.
Furocort and other medicines
Tell your doctor or pharmacist about all medicines you are taking now or have recently taken, as well as any medicines you plan to take, including those available without a prescription. It is especially important to tell your doctor about any of the following medicines you are currently taking or have recently taken:
- steroids in tablets or injections
- steroids in creams
- medicines used in asthma
- ritonavir or cobicistat used in the treatment of HIV
- ketoconazole used in the treatment of fungal infections
Your doctor will assess whether you can use Furocort with these medicines. Some of these medicines may increase the side effects of Furocort, and your doctor may want to monitor your condition closely while taking such medicines.
Do not use Furocort at the same time as other nasal sprays containing steroids.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor before using this medicine.
Do not use Furocort if you are pregnant, or plan to have a child, unless your doctor or pharmacist advises you to.
Do not use Furocort if you are breastfeeding, unless your doctor or pharmacist advises you to.
Driving and using machines
It is unlikely that Furocort will affect your ability to drive or use machines.
Furocort contains benzalkonium chloride
This medicine contains 8.25 micrograms of benzalkonium chloride per dose (27.5 micrograms).
Benzalkonium chloride may cause irritation or swelling inside the nose, especially if used for a long time. Tell your doctor or pharmacist if you experience discomfort while using the spray.
3. How to use Furocort
Always use this medicine exactly as your doctor or pharmacist has told you. Do not use more than the recommended dose. If you are not sure, ask your doctor or pharmacist.
When to use Furocort
- Use once a day.
- Use at the same time every day.
The medicine treats symptoms both during the day and at night.
How long does Furocort work
Some people may not feel an improvement even after a few days of using Furocort. The effect of the medicine is usually observed within 8 to 24 hours of use.
What dose to use
Adults, adolescents, and children over 12 years
- The usual starting dose is 2 sprays in each nostril once a day.
- After symptoms are under control, the dose can be reduced to 1 spray in each nostril once a day.
Children aged 6 to 11 years
- The usual starting doseis 1 spray in each nostril once a day.
- If symptoms are severe, your doctor may recommend increasing the dose to 2 sprays in each nostril once a day until symptoms are under control. Then the dose can be reduced to 1 spray in each nostril once a day.
How to use the nasal spray
Furocort has no taste or smell. It is sprayed into the nose in the form of a mist. Be careful not to get the spray into your eyes. If this happens, rinse your eyes with water.
In this leaflet, after section 6, there is a detailed instruction for using the nasal spray. Follow these instructions carefully to get the most benefit from using Furocort.
- See: Detailed instructions for using the nasal spray, after section 6.
Using a higher dose of Furocort than recommended
Contact your doctor or pharmacist.
Missing a dose of Furocort
If you miss a dose, take it as soon as you remember.
If it is almost time for your next dose, wait and take it at that time. Do not take a double dose to make up for a missed dose.
If you have any further questions about using this medicine or experience discomfort while using the nasal spray, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Allergic reactions: seek medical help immediately
Allergic reactions to Furocort are rare and affect less than 1 in 1,000 people.
In a small number of patients, allergic reactions can develop into a serious condition, even life-threatening, if not treated. Symptoms include:
- wheezing, coughing, or difficulty breathing
- sudden weakness or dizziness (which may lead to fainting or loss of consciousness)
- swelling of the face
- rash or redness of the skin.
In many cases, these symptoms will be signs of less severe side effects. However, they can be serious- so if you experience any of these symptoms:
Seek medical help immediately.
Very common side effects(occurring in more than 1 in 10 people):
- Nosebleeds (usually mild), especially if Furocort has been used continuously for more than 6 weeks.
Common side effects(occurring in less than 1 in 10 people)
- Ulcers of the nasal mucosa - which can cause irritation or discomfort inside the nose. There may also be visible blood streaks when blowing your nose.
- Headache
- Shortness of breath.
Uncommon side effects(occurring in less than 1 in 100 people):
- Pain, burning, irritation, tenderness, or dryness inside the nose.
Rare side effects(occurring in less than 1 in 10,000 people):
- Small holes (perforations) in the nasal septum, separating the nostrils.
Side effects of unknown frequency(frequency cannot be estimated from available data)
- Growth retardation in children.
- Blurred vision or transient vision disturbances during long-term use
- Chest tightness causing breathing difficulties.
- Voice disorders, loss of voice
- Taste disorders, loss of taste, loss of smell
Nasal corticosteroids may affect the production of hormones in the body, especially if large doses are used for a long time. This side effect may cause growth retardation in children.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C, 02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Furocort
Keep the medicine out of the sight and reach of children.
The protective cap should always be on the actuator after use.
Do not use this medicine after the expiry date stated on the packaging after EXP.
The expiry date refers to the last day of the month.
The shelf life after first opening of Furocort, nasal spray is 2 months.
Do not store in the refrigerator or freeze.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the packaging and other information
What Furocort contains
- The active substance is fluticasone furoate. Each actuation releases 27.5 micrograms of fluticasone furoate.
- The other ingredients are: glucose, microcrystalline cellulose (E460), sodium carmellose (E466), polysorbate 80 (E433), benzalkonium chloride, disodium edetate, water for injections (see section 2).
What Furocort looks like and contents of the pack
The medicine is a white suspension for nasal spray in a brown glass bottle with a dosing pump (polypropylene/aluminum), applicator, and protective cap made of polypropylene in a cardboard box. Furocort is available in a pack size of 120 doses.
Marketing authorization holder and manufacturer
Marketing authorization holder
Teva B.V.
Swensweg 5, 2031 GA Haarlem
Netherlands
Manufacturer
Teva Czech Industries s.r.o.
Ostravska 305/29
747 70 Opava – Komarov, Czech Republic
Teva Pharmaceuticals Polska Sp. z o.o., ul. Emilii Plater 53, 00-113 Warsaw, tel. (22) 345 93 00.
This medicine is authorized in the Member States of the European Economic Area under the following names:
Netherlands
Fluticasonfuroaat ratiopharm 27,5 microgram/dosis, neusspray suspensie
Bulgaria
Фурокорт 27,5 микрограма на впръскване спрей за нос, суспензия
Spain
Fluticasona Teva 27,5 microgramos/pulverización, suspensión para pulverización nasal
Poland
Furocort
Date of last revision of the leaflet: March 2025
DETAILED INSTRUCTIONS FOR USING THE NASAL SPRAY
What the nasal spray looks like
The nasal spray is in a brown glass bottle with a dosing pump, applicator, and protective cap. It contains 120 doses.
Five important things to know about using the nasal spray
- Furocort is in a brown glass bottle. To check how much medicine is left in the bottle, place the bottle against a bright light source. The level of the medicine in the bottle will be visible.
- When using the nasal spray for the first time, shake the bottle vigorouslywith the protective cap on the actuator for about 10 seconds. This is important because Furocort is a thick suspension that becomes liquid after vigorous shaking. The medicine will only spray if it is liquid.
- Press the pump firmly to release the mist through the actuator tip.
- Always put the protective cap back on the actuatorwhen not using the nasal spray. The protective cap prevents dust from entering, maintains the pressure in the sprayer, and prevents the actuator tip from clogging.
- Never use a pinor other sharp object to clean the actuator tip. This can damage the dosing mechanism.
Preparing the nasal spray for use
You need to prepare the nasal spray:
- before using it for the first time
- after leaving the actuator without the protective cap for 5 days or when the device has not been used for 30 days or longer.
Preparing the nasal spray helps ensure that you always get a full dose of the medicine.
Follow these steps:
- 1. Shake the bottle vigorouslywith the protective cap on the actuator for about 10 seconds.
- 2. Remove the protective cap gently - see Figure 1.
Figure 1
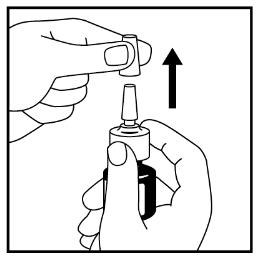
- 3. Hold the nasal spray upright, tilt it, and then point the actuator away from you.
- 4. Activate the dosing system by placing two fingers on either side of the pump and holding the bottom of the bottle with your thumb. Press and release the pump. Press at least 6 times until a mist is released into the air - see Figure 2.
Figure 2
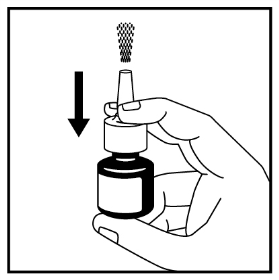
- 5. The nasal spray is now ready to use.
Using the nasal spray
- 1. Shakethe nasal spray vigorously.
2. Remove the protective cap.
- 3. Blow your noseto clear your nostrils, then tilt your head slightly forward.
- 4. Place the actuator tip in one nostril, closing the other nostril with your finger - see Figure 3. Point the actuator tip slightly towards the outer part of the nose, away from the nasal septum. This will allow the medicine to reach the right part of the nose.
- 5. While inhaling through your nose, press the pump firmly to release the dose.
Figure 3
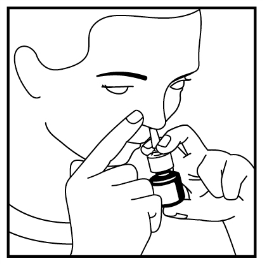
- 6. Remove the actuator from your nostril and exhale through your mouth.
- 7. If your doctor has prescribed 2 sprays in each nostril, repeat steps 4 to 6.
- 8. To administer the medicine to the other nostril, repeat steps 4 to 7.
- 9. Put the protective cap back on the nasal spray.
Cleaning the nasal spray
After each use:
- 1. Wipe the actuator tip and the inside of the protective cap with a dry, clean tissue.
- 2. Do not use water to clean the actuator tip.
- 3. Never use a pin or other sharp object to unclog the actuator tip.
- 4. Always put the protective cap back on the nasal spray after use.
If the nasal spray does not work:
- Check if there is still medicine left. Look at the level of the medicine through the bottle. If there is very little medicine left, the nasal spray may not work properly.
- Check if the nasal spray container is damaged.
- If you suspect that the actuator tip is clogged, never use a pin or other sharp object to unclog it.
- Try to prime it again by following the instructions in the "Preparing the nasal spray for use" section.
- If the nasal spray still does not work, or if a stream of liquid comes out, take the nasal spray to a pharmacy for advice.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterTeva Czech Industries s.r.o.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FurocortDosage form: Aerosol, 27.5 mcg/doseActive substance: fluticasone furoatePrescription requiredDosage form: Aerosol, 27.5 mcg/doseActive substance: fluticasone furoatePrescription requiredDosage form: Aerosol, 50 mcg/doseActive substance: mometasoneManufacturer: FARMEA US Pharmacia Sp. z o.o.Prescription not required
Alternatives to Furocort in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Furocort in Spain
Alternative to Furocort in Ukraine
Online doctors for Furocort
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Furocort – subject to medical assessment and local rules.








