
ALDARA 5% CREAM


How to use ALDARA 5% CREAM
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Aldara 5% Cream
Imiquimod
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
|
Contents of the pack and other information
- What is Aldara cream and what is it used for.
- What you need to know before you use Aldara cream.
- How to use Aldara cream.
- Possible side effects
- Storage of Aldara cream.
Contents of the pack and other information.
1. What is Aldara cream and what is it used for
Aldara cream is indicated for three different diseases. Your doctor may prescribe Aldara cream for the treatment of:
- Warts (genital warts) on the surface of the genitals (sex organs) and around the anus (back passage).
- Superficial basal cell carcinoma.
This is a common type of slow-growing skin cancer that is very unlikely to spread to other parts of the body. It usually appears in older and middle-aged people, especially those with fair skin due to excessive sun exposure. If left untreated, basal cell carcinoma can cause disfigurement, especially on the face, so early detection and treatment are essential.
- Actinic keratosis
Actinic keratosis consists of rough areas of skin found in people who have been exposed to a lot of sunlight during their lifetime. Some are colored, others are grayish, pink, red, or brown. They can be smooth and scaly, or thick, hard, and wart-like. Aldara should be used exclusively for smooth actinic keratosis on the face and scalp of patients with a healthy immune system when your doctor decides that Aldara is the most appropriate treatment.
Aldara cream helps your body's own immune system to produce natural substances that help fight your basal cell carcinoma, actinic keratosis, or the virus that has caused the wart.
2. What you need to know before you use Aldara cream
Do not use Aldara cream
- if you are allergic to imiquimod or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor or pharmacist before you start using Aldara cream.
- If you have used Aldara cream or similar preparations before, you should talk to your doctor before starting this treatment.
- If you have autoimmune disorders.
- If you have had an organ transplant.
- Do not use Aldara cream until the area to be treated has healed after previous drug or surgical treatment.
- Avoid contact with the eyes, lips, and nostrils. In case of accidental contact, remove the cream by washing with water.
- Do not apply the cream internally.
- Do not use more cream than your doctor advises.
- Do not cover the treated area with bandages or other dressings after applying Aldara cream.
- If the treated area becomes too irritated, remove the cream with mild soap and water. As soon as the problem has resolved, you can reapply the cream.
- Tell your doctor if you have changes in your blood count.
Due to the way Aldara works, there is a possibility that the cream may worsen existing inflammation in the treatment area.
- If you are being treated for genital warts, follow these additional precautions:
Men with warts under the foreskin should pull the foreskin back every day and wash underneath. If the foreskin is not washed daily, it is more likely that signs of tightness, swelling, and skin peeling will occur, and as a result, it will be more difficult to pull the foreskin back. If these symptoms occur, stop treatment immediately and contact your doctor.
If you have open sores: do not start using Aldara cream until the sores have completely healed.
If you have internal warts: do not use Aldara cream in the urethra (the opening through which urine passes), vagina (birth canal), cervix (internal female organ), or any area inside the anus (rectum).
Do not use this medication for more than one treatment cycle if you have immune system problems, either due to a disease or medications you are taking. If you think this may be the case, consult your doctor.
If you are infected with HIV (AIDS), you should inform your doctor, as it has not been demonstrated that treatment with Aldara cream is equally effective in these patients.
If you decide to have sex while you still have warts, apply Aldara after, never before, sex. Aldara cream can weaken condoms and diaphragms, so the cream should not be left on during sexual activity. Remember that Aldara cream does not protect against the transmission of HIV or other sexually transmitted diseases to other people.
- If you are being treated for basal cell carcinoma or actinic keratosis, follow these additional precautions:
Do not use sunlamps or tanning devices and avoid sunlight as much as possible during treatment with Aldara cream. When going outside, use protective clothing and wide-brimmed hats.
During the use of Aldara cream and until healing, it is likely that the treated area will look noticeably different from normal skin.
Children and adolescents
It is not recommended for use in children and adolescents.
Other medicines and Aldara cream
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
No medicines are known to be incompatible with Aldara cream.
Pregnancy and breastfeeding
Talk to your doctor or pharmacist before you use any medicine.
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine. Your doctor will assess the risks and benefits of using Aldara cream during pregnancy. Animal studies do not indicate direct or indirect harmful effects with respect to pregnancy.
Do not breastfeed while using Aldara cream, as it is not known whether imiquimod is excreted in breast milk.
Driving and using machines
This medicine has a negligible influence on the ability to drive and use machines.
Aldara cream contains methyl hydroxybenzoate, propyl hydroxybenzoate, cetyl alcohol, stearic alcohol, and benzyl alcohol.
Methyl hydroxybenzoate (E 218) and propyl hydroxybenzoate (E 216) may cause allergic reactions (possibly delayed). Cetyl alcohol and stearic alcohol may cause local skin reactions (such as contact dermatitis).
This medicine contains 5 mg of benzyl alcohol in each sachet. Benzyl alcohol may cause allergic reactions and moderate local irritation.
3. How to use Aldara cream
Children and adolescents:
It is not recommended for use in children and adolescents.
Adults:
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are not sure, talk to your doctor or pharmacist.
Wash your hands carefully before and after applying the cream. Do not cover the treated area with bandages or other dressings after applying Aldara cream.
Open a new sachet each time you apply the cream. Discard any cream left in the sachet after use. Do not save the opened sachet for use another day.
The frequency and duration of treatment vary for genital warts, basal cell carcinoma, and actinic keratosis (see specific instructions for each indication).
Instructions for Application of Aldara Cream
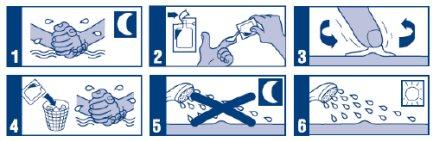
- If you are being treated for genital warts:
Instructions for correct use of the medication: (Tuesday, Thursday, and Saturday)
- Before bedtime, wash your hands and the treatment area with mild soap and water. Dry well.
- Open a new sachet and place the cream on the tip of your finger.
- Apply a thin layer of Aldara cream to a clean and dry wart area and spread it smoothly over the skin until the cream disappears.
- After applying the cream, discard the opened sachet and wash your hands with water and soap.
- Let the cream act on the warts for 6 to 10 hours. During this time, do not shower or bathe.
- After 6-10 hours, wash the area where you applied the cream with water and mild soap.
Apply Aldara 3 times a week. For example, apply the cream on Tuesdays, Thursdays, and Saturdays. One sachet contains enough cream to cover a wart area of 20 cm2.
Men who are treating warts located under the foreskin should pull the foreskin back and wash the area every day (see section 2 "Warnings and precautions").
Continue using Aldara cream as directed until your warts have completely disappeared (in half of the women whose warts disappear, this happens within 8 weeks; in half of the men whose warts disappear, this happens within 12 weeks. However, in some patients, warts may disappear within 4 weeks).
Do not use Aldara cream for more than 16 weeks for the treatment of each episode of warts.
If you think the action of Aldara cream is too strong or too weak, talk to your doctor or pharmacist.
- If you are being treated for basal cell carcinoma:
Instructions for correct use of the medication (Monday, Tuesday, Wednesday, Thursday, and Friday)
- Before bedtime, wash your hands and the treatment area with mild soap and water. Dry well.
- Open a new sachet and place a small amount of cream on the tip of your finger.
- Apply Aldara cream to the affected area and 1 cm around the affected area (approximately one finger). Spread it smoothly over the skin until the cream disappears.
- After applying the cream, discard the opened sachet and wash your hands with water and soap.
- Let Aldara cream act on the skin for about 8 hours. During this time, do not shower or bathe.
- After about 8 hours, wash the area where you applied Aldara cream with mild soap and water.
Apply enough Aldara cream to cover the treatment area and 1 cm around the treatment area, once daily, for 5 consecutive days each week, for 6 weeks. For example, apply the cream from Monday to Friday. Do not apply the cream on Saturday or Sunday.
- If you are being treated for actinic keratosis:
Instructions for correct use of the medication: (Tuesday, Thursday, and Saturday)
- Before bedtime, wash your hands and the treatment area with mild soap and water. Dry well.
- Open a new sachet and place a small amount of cream on the tip of your finger.
- Apply Aldara cream to the affected area. Spread it smoothly over the area until the cream disappears.
- After applying the cream, discard the opened sachet and wash your hands with water and soap.
- Let Aldara cream act on the skin for about 8 hours. During this time, do not shower or bathe.
- After about 8 hours, wash the area where you applied Aldara cream with mild soap and water.
Apply Aldara cream 3 times a week. For example, apply the cream on Tuesdays, Thursdays, and Saturdays. One sachet contains enough cream to cover an area of 25 cm2. Continue treatment for 4 weeks. Four weeks after finishing the first treatment, your doctor will assess your skin. If not all lesions have disappeared, another 4 weeks of treatment may be necessary.
If you use more Aldara cream than you should
Remove the excess with water and mild soap. When the skin reaction has disappeared, you can continue treatment.
In case of accidental ingestion of Aldara cream, talk to your doctor.
If you forget to use Aldara cream
If you miss a dose, apply the cream as soon as you remember and continue your usual routine.
Do not apply the cream more than once a day.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
The frequencies of adverse effects are defined as:
Very frequent adverse effects (observed in more than 1 in 10 patients).
Frequent adverse effects (observed in less than 1 in 10 patients).
Infrequent adverse effects (observed in less than 1 in 100 patients).
Rare adverse effects (observed in less than 1 in 1,000 patients).
Very rare adverse effects (observed in less than 1 in 10,000 patients).
Like all medicines, this medicine can cause adverse effects, although not all people will experience them.
Consult your doctor or pharmacist if you do not feel well during the administration of Aldara cream.
Some patients have presented with skin discoloration in the area where Aldara was applied. Although these changes tend to improve over time, they could be permanent in some patients. If your skin presents an adverse reaction when using Aldara cream, interrupt the application of the cream, wash the area with water and mild soap, and contact your doctor or pharmacist.
In some individuals, a decrease in blood counts has been detected. A decrease in blood counts can make you more susceptible to infections, cause bruising more easily, or cause fatigue. If you notice any of these symptoms, inform your doctor.
Some patients with autoimmune disorders may experience worsening of their disease. Consult your doctor if you experience any changes during treatment with Aldara cream.
In rare cases, severe dermatological reactions have occurred. Interrupt treatment with Aldara and inform your doctor immediately if you notice skin lesions or spots on the skin that start as small red areas and evolve into small targets, possibly with inflammation, fever, feeling of general discomfort, visual problems, burning, swollen or painful eyes, and swollen mouth.
A reduced number of patients have experienced hair loss in the treated area or in the surrounding area.
- If you are being treated for genital warts:
Many of the adverse effects of Aldara cream are due to its local action on the skin.
The very frequenteffects include redness (61% of patients), skin scaling (30% of patients), and formation of crusts and swelling. Hardening under the skin, small open sores, scabs formed during healing, or small blisters under the skin may also occur. You may also feel itching (32% of patients), burning sensation (26% of patients), or pain in the areas where you apply Aldara cream (8% of patients). Most of these skin reactions are moderate, and the skin returns to a normal appearance in approximately two weeks after finishing treatment.
Frequently, some patients (4% or less) have experienced headache, infrequentlyfever, flu-like symptoms, joint and muscle pain, uterine prolapse, pain during intercourse in women, erection difficulties, increased sweating, dizziness, stomach and intestinal symptoms, ringing in the ears, flushing, fatigue, vertigo, migraine, tingling, insomnia, depression, loss of appetite, inflammation of the glands, bacterial, viral, and fungal infections (e.g., cold sores), vaginal infection (including thrush), cough, and colds with sore throat.
Very rarely, severe and painful reactions have occurred, particularly when more cream than recommended has been used. Painful skin reactions at the vaginal opening have rarely caused some women to have difficulty urinating. If this happens, seek medical attention immediately.
- If you are being treated for basal cell carcinoma:
Many of the adverse effects of Aldara cream are due to its local action on your skin. Local skin reactions may be a sign that the drug is working as expected.
Very frequently, the treated skin may present with mild itching.
Frequent effectsare: tingling, small inflamed areas on the skin, pain, burning, irritation, bleeding, redness, or rash. If any skin reaction becomes too bothersome during treatment, consult your doctor. They may advise you to stop applying Aldara cream for a few days (i.e., a brief break from treatment). If pus or other signs of infection appear, inform your doctor. Apart from skin reactions, other common effects are inflammation of the glands and back pain.
Infrequently, some patients experience changes at the application site (discharge, inflammation, swelling, crust formation, cracked skin, blisters, dermatitis) or irritability, dizziness, dry mouth, flu-like symptoms, and fatigue.
- If you are being treated for actinic keratosis
Many of the adverse effects of Aldara cream are due to its local action on your skin. Local skin reactions may be a sign that the drug is working as expected.
Very frequently, the treated skin may present with mild itching.
Frequent effectsinclude: pain, burning, irritation, or redness.
If any skin reaction becomes too bothersome during treatment, consult your doctor. They may advise you to stop applying Aldara cream for a few days (i.e., a brief break from treatment).
If pus or other signs of infection appear, inform your doctor. Apart from skin reactions, other frequent effects are headache, anorexia, nausea, muscle and joint pain, and fatigue.
Infrequently, some patients experience changes at the application site (bleeding, inflammation, discharge, sensitivity, swelling, small inflamed areas on the skin, tingling, crust formation, scarring, ulceration, or feeling of heat or discomfort), or inflammation of the skin covering the nose, nasal obstruction, flu or flu-like symptoms, depression, eye irritation, eyelid inflammation, sore throat, diarrhea, actinic keratosis, redness, facial swelling, ulcers, limb pain, fever, weakness, or tremors.
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect not listed in this prospectus. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Aldara Cream
Keep this medicine out of the sight and reach of children.
Do not store above 25°C.
Do not use this medicine after the expiration date stated on the outer packaging and on the label after EXP.
Once the sachets are opened, they should not be reused.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Package Contents and Additional Information
Aldara Cream Composition
- The active ingredient is imiquimod. Each sachet contains 250 mg of cream (100 mg of cream contains 5 mg of imiquimod).
- The other ingredients are isostearic acid, benzyl alcohol, cetyl alcohol, stearyl alcohol, white petrolatum, polysorbate 60, sorbitan stearate, glycerol, methyl hydroxybenzoate (E 218), propyl hydroxybenzoate (E 216), xanthan gum, and purified water (see also section 2 "Aldara cream contains methyl hydroxybenzoate, propyl hydroxybenzoate, cetyl alcohol, stearyl alcohol, and benzyl alcohol").
Product Appearance and Package Contents
- Each Aldara 5% cream sachet contains 250 mg of a white or yellowish cream.
- Each package contains 12 or 24 single-use polyester/paper/aluminum sachets. Only certain package sizes may be marketed.
Marketing Authorization Holder
Viatris Healthcare Limited
Damastown Industrial Park
Mulhuddart
Dublin 15
DUBLIN
Ireland
Manufacturer
Swiss Caps GmbH
Grassingerstraße 9
83043 Bad Aibling
Germany
You can request more information about this medicine by contacting the local representative of the marketing authorization holder.
België/Belgique/Belgien Mylan EPD bvba/sprl Terhulpsesteenweg, 6A B-1560 Hoeilaart Tél/Tel: +32 2 658 61 00 | Luxembourg/Luxemburg Mylan EPD bvba/sprl Terhulpsesteenweg, 6A B-1560 Hoeilaart Tél/Tel: +32 2 658 61 00 |
България Милан ЕПД ЕООД тел.: +359 2 44 55 400 | Magyarország Mylan EPD Kft. 1138 Budapest Váci út 150 Tel: +36 1 465 2100 |
Česká republika Viatris CZ s.r.o. Tel: +420 222 004 400 | Malta V.J. Salomone Pharma Limited Upper Cross Road Marsa, MRS 1542 Tel: +356 21 22 01 74 |
Danmark Viatris ApS Borupvang 1 2750 Ballerup Tlf: +45 28 11 69 32 | Nederland Mylan Healthcare B.V. Krijgsman 20 1186 DM Amstelveen Tel: +31 (0)20 426 3300 |
Deutschland Viatris Healthcare GmbH Lütticher Straße 5 53842 Troisdorf Tel: +49 800 0700 800 | Norge Viatris AS Hagaløkkveien 26 1383 Asker Tlf: +47 66 75 33 00 |
Eesti Meda Pharma SIA Liivalaia 13/15 11018 Tallinn Tel: +372 62 61 025 | Österreich Mylan Österreich GmbH Guglgasse 15 1110 Wien Tel: + 43 (0)1 86 390 0 |
Ελλάδα Viatris Hellas Ltd Τηλ: +30 210 010 0002 | Polska Mylan Healthcare Sp. z o.o. ul. Postepu 21B 02-676 Warszawa Tel: +48 22 546 6400 |
España Viatris Pharmaceuticals, S.L.U. Tel: +34 900 102 712 | Portugal Viatris Healthcare, Lda. Av. D. João II, Edifício Atlantis, nº 44C – 7.3 e 7.4 1990-095 Lisboa Tel: +351 214 127 200 |
France Viatris Médical 1 bis place de la Défense – Tour Trinity 92400 Courbevoie Tél: +33 (0) 1 40 80 15 55 | România BGP PRODUCTS SRL Tel.: +40372 579 000 |
Hrvatska Viatris Hrvatska d.o.o. Koranska 2 10 000 Zagreb Tel: +385 1 2350 599 | Slovenija Viatris d.o.o. Tel: +386 1 23 63 180 |
Ireland Mylan Ireland Limited Tel: +353 1 8711600 | Slovenská republika Viatris Slovakia s.r.o. Tel: +421 2 32 199 100 |
Ísland Icepharma hf. Sími: +354 540 8000 | Suomi/Finland Viatris Oy Vaisalantie 2-8/Vaisalavägen 2-8 02130 Espoo/Esbo Puh/Tel: +358 20 720 9555 |
Italia Mylan Italia Via Vittor Pisani, 20 20124 Milano Tel: +39 0261246921 | Sverige Viatris AB Box 23033 104 35 Stockholm +46 (0) 8 630 19 00 |
Κύπρος Βαρνάβας Χατζηπαναγής Λτδ Λεωφ. Γιάννου Κρανιδιώτη 226 ΤK 2234, Λατσιών, Λευκωσία Τηλ.: +357 22207700 | United Kingdom(Northern Ireland) Mylan IRE Healthcare Limited Tel: +353 18711600 |
Latvija Meda Pharma SIA 101 Mukusalas str. Riga LV-1004 Talr: +371 67616137 | |
Lietuva Meda Pharma SIA Žalgirio str. 90-100 Vilnius LT-09303 Tel. + 370 52051288 |
Date of Last Revision of this Prospectus: {MM/YYYY}
Detailed information about this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ALDARA 5% CREAMDosage form: CREAM, 50 mg/gActive substance: imiquimodManufacturer: Viatris Healthcare LimitedPrescription requiredDosage form: CREAM, 5 %Active substance: imiquimodManufacturer: Mibe Pharma Espana S.L.Prescription requiredDosage form: CREAM, 50 mg/gActive substance: imiquimodManufacturer: Industrial Farmaceutica Cantabria S.A.Prescription required
Online doctors for ALDARA 5% CREAM
Discuss questions about ALDARA 5% CREAM, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions








