
ACTOCORTINA 75 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use ACTOCORTINA 75 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
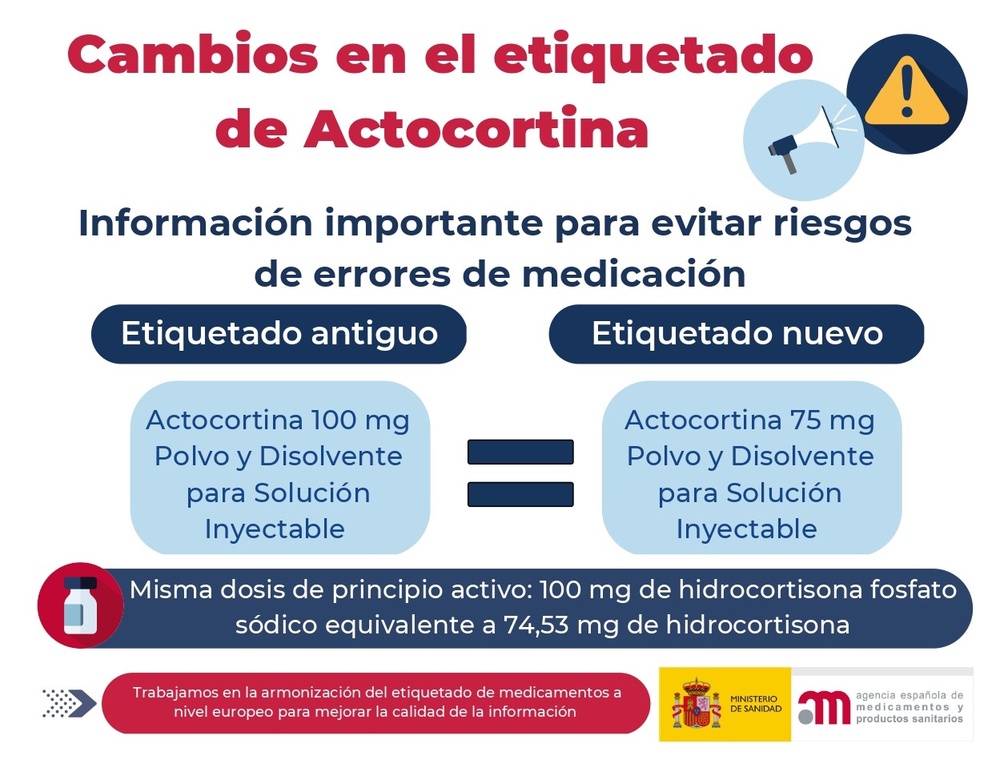
Actocortina 75 mg Powder and Solvent for Solution for Injection
Hydrocortisone
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you., as it contains important information for you.
|
Contents of the Package Leaflet
- What is Actocortina and what is it used for
- What you need to know before you use Actocortina
- How to use Actocortina
- Possible side effects
- Storage of Actocortina
- Contents of the pack and further information
1. What is Actocortina and what is it used for
Actocortina belongs to a group of hormonal preparations called
systemic corticosteroids and acts as potent anti-inflammatory agents.
Actocortina is used for the treatment of:
- acute exacerbations of asthma
- anaphylactic shock and acute hypersensitivity reactions that constitute a danger to the patient's life (e.g. angioedema, laryngeal edema)
- acute adrenal insufficiency
- treatment of acute organ transplant rejection
- myxedema coma
- necrotizing vasculitis
- rheumatoid arthritis
tenosynovitis and bursitis
2. What you need to know before you use Actocortina
Do not use Actocortina
- if you are allergic to the active substance, other steroids or any of the other ingredients of this medicine (listed in section 6).
Except in emergency situations, this medicine should not be used in the following cases, as there is a risk of worsening. This decision should be made by your doctor:
- gastric or duodenal ulcers
- known psychiatric disorders, emotional instability, psychosis
- glaucoma
- herpetic keratitis
- lymphadenopathy following tuberculosis vaccination
- amebic or fungal infections
- poliomyelitis
- viral diseases such as chickenpox, herpes simplex, herpes zoster
- latent or manifest tuberculosis
- during the pre- and post-vaccination period with live virus or attenuated bacterial vaccines (approximately 8 weeks before and 2 weeks after vaccination)
Warnings and precautions
Consult your doctor or pharmacist before starting treatment with Actocortina.
You should inform your doctor if you have or have had any of the following disorders:
- renal insufficiency
- heart failure
- diabetes mellitus
- osteoporosis
- hypertension
- glaucoma
- epilepsy
- severe psychiatric disorders
- muscle injury caused by steroid treatment
- gastrointestinal diseases such as peptic ulcer, ulcerative colitis, ileitis, diverticulitis
- myasthenia gravis
- poliomyelitis
- liver failure
- severe fungal infections
- tuberculosis
- known or suspected infection (viral, bacterial or amebic).
- pre-existing lymphoid tumors
- hyperparathyroidism
- if you have hyperactivity of the thyroid gland (hyperthyroidism)
- hypothyroidism
- cerebral malaria
Contact your doctor if you experience blurred vision or other visual disturbances.
Rapid intravenous injection of high doses of Actocortina can sometimes cause acute cardiac problems, so injections should be administered slowly or by infusion.
This medicine increases the risk of infections and increases their severity. It is recommended to rule out infections such as chickenpox or measles before starting treatment. It can also increase the risk of eye infections.
Live vaccines should not be administered to patients being treated with corticosteroids, and the response to other types of vaccines may be diminished.
Long-term systemic therapy with Actocortina can cause adrenal insufficiency, Cushing's syndrome or unbalanced sugar levels in the body.
Actocortina can cause mental disorders, including euphoria, insomnia, mood changes, personality changes, depression, and psychotic tendencies. This can occur during the start of treatment and during dose adjustments. The risks may be greater when high doses are administered. Most reactions resolve after dose reduction.
Treatment with high doses of Actocortina can cause benign "steroid diabetes". This is reversible when therapy is stopped. In diabetics, treatment usually causes dysregulation, which can be compensated by adjusting the insulin dose.
Contact your doctor immediately if you experience muscle weakness, muscle pain, cramps, and stiffness while using hydrocortisone. They may be symptoms of a condition called thyrotoxic periodic paralysis, which can occur in patients with hyperactivity of the thyroid gland (hyperthyroidism) treated with hydrocortisone. You may need additional treatment to alleviate this condition.
Children and adolescents
It can cause growth retardation in childhood and adolescence, which may be irreversible.
In premature infants, it can cause hypertrophic cardiomyopathy. If hydrocortisone is administered to a premature newborn, it may be necessary to monitor heart function and structure.
Elderly patients
This medicine can have greater consequences in these patients, such as osteoporosis, hypertension, hypokalemia, diabetes, increased risk of infections, and skin thinning. Close medical supervision is required to avoid life-threatening reactions.
Other medicines and Actocortina
Tell your doctor if you are taking, have recently taken, or might take any other medicines.
The following medicines reduce the therapeutic effect of Actocortina:
- rifampicin and rifabutin (drugs used to treat mycobacterial infections such as tuberculosis)
- carbamazepine (drug used to treat manic-depressive disorders), barbiturates (phenobarbital, drug used to treat epilepsy)
- phenytoin (drug used to treat seizures and convulsions)
- ephedrine (drug with bronchodilator effect)
- aminoglutethimide (drug used to treat adrenal cortex tumors)
- cholestyramine and colestipol (drugs used to treat hypercholesterolemia)
- CYP3A4 inducers (e.g. rifampicin, carbamazepine, phenytoin, dexamethasone)
The following medicines can increase the therapeutic effect of Actocortina:
- cyclosporin (immunosuppressive drug)
- oral contraceptives (e.g. estrogens)
- drugs for the treatment of HIV (ritonavir, cobicistat)
Actocortina reduces the effects of the following medicines:
- hypoglycemic agents (drugs used to treat diabetes, including insulin)
- antihypertensive agents (drugs used to treat high blood pressure)
- diuretics (drugs used to increase urine production)
- anticholinesterases (drugs used to treat muscle weakness and to diagnose gallstones)
Actocortina can increase the effects of the following medicines:
- acetazolamide, loop diuretics, amphotericin B, and carbenoxolone (concomitant treatment may cause subsequent cardiac arrhythmias)
- coumarin anticoagulants (concomitant treatment requires monitoring to avoid spontaneous bleeding)
- cyclosporin (immunosuppressive drugs)
Other interactions with medicines
Actocortina can interact with other medicines, so your doctor will perform thorough checks if you are taking these medicines.
Actocortina increases the toxicity of digitalis glycosides (e.g. digoxin) and the risk of gastrointestinal bleeding and ulceration with concomitant use of medicines such as aspirin and non-steroidal anti-inflammatory drugs (NSAIDs).
Concomitant use of Actocortina with fluoroquinolones (drugs used to treat bacterial infections) can increase the risk of tendon rupture.
Corticosteroids can reduce the effects of certain types of vaccines.
Using Actocortina with food
Licorice can increase the risk of side effects of Actocortina.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine.
Pregnancy
Actocortina should not be used during pregnancy, nor in women of childbearing potential who are not using contraceptive methods.
Breastfeeding
Actocortina is excreted in breast milk. A risk to the newborn or breastfed child cannot be excluded.
Your doctor will decide whether to discontinue breastfeeding or discontinue treatment.
Driving and using machines
No studies on the effects on the ability to drive and use machines have been performed.
Drug analysis/anti-doping control
Athletes are informed that this medicine contains a component that can result in a positive doping control test.
Actocortina contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per vial; i.e. it is essentially "sodium-free".
3. How to use Actocortina
Your doctor will decide on the injection site, the amount of medicine, and the number of injections to be administered, depending on the disease to be treated and its severity. Your doctor will administer the lowest effective dose for the shortest duration necessary to alleviate the symptoms.
Your doctor or another healthcare professional will administer the treatment. The contents of the vial should be dissolved with water for injection and administered by intramuscular, slow intravenous, drop-by-drop, or continuous infusion injection.
The recommended dose is as follows:
Adults
- Intravenous or intramuscular:
Generally, the recommended single dose range, although not limited, varies from a fraction of the vial contents (less than 74.53 mg of hydrocortisone) to approximately 372.65 mg of hydrocortisone administered by slow intravenous injection over a period of 1 to 10 minutes (for doses of 372.65 mg or higher). This dose may be repeated at intervals of 2, 4, or 6 hours, depending on the disease being treated and the patient's response. Alternatively, this medicine can be administered as an intravenous infusion. A clinical effect is observed within 2 to 4 hours and persists for up to 8 hours after intravenous injection. The same dose can be administered by intramuscular injection, but the response is likely to be slower, especially in shock. The dose should not exceed 6 g/day.
- Intra-articular and soft tissue (infiltration when limited to one or two locations):
The amount administered depends on the site of application (large joints: 19 mg; small joints: 7.5 mg; serous bursae, including hallux valgus: 19-28 mg; tendon sheaths: 4-9 mg; infiltration into soft tissues: 19-37.5 mg; ganglia: 9-19 mg).
Use in children
Although the dose of Actocortina may be reduced for infants and children, it is governed more by the severity of the condition and the response than by age or body weight, but should not be less than 25 mg per day.
Elderly patients
If you use Actocortina for a prolonged period, you will have an increased risk of diabetes, hypertension, congestive heart failure, osteoporosis, and depression.
Close clinical monitoring is required to avoid life-threatening reactions.
Patients with impaired renal function
If you have severe kidney problems, your doctor may monitor you and may need to adjust the dose.
Patients with impaired liver function
You are more likely to experience serious unwanted effects. Dose adjustments may be necessary.
If you use more Actocortina than you should
In prolonged treatments, an increase in cortisol levels in the blood may occur, which subsides within a few days after discontinuation of the medication.
No cases of overdose have been reported. In case of overdose or accidental ingestion, consult your doctor and/or hospital.
If you forget to use Actocortina
Do not take a double dose to make up for forgotten doses.
If you stop using Actocortina
Your doctor will indicate the duration of your treatment with Actocortina.
Treatment should not be stopped abruptly. Your doctor will indicate how to do it, as rapid withdrawal of Actocortina after prolonged treatment can lead to important complications such as acute adrenal insufficiency, hypotension, hormonal disturbances, or death.
A "withdrawal syndrome" may also occur, including fever, myalgia, arthralgia, rhinitis, conjunctivitis, painful/pruritic skin nodules, and weight loss.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
The adverse effects of Actocortina depend on the dose, the time of administration, and the duration of treatment. Adverse reactions can be minimized by using the lowest effective dose for the shortest possible time.
The following adverse drug reactions have been reported with hydrocortisone administered at high doses or for indications other than replacement therapy in adrenal insufficiency, although the frequency is unknown.
Organs/Systems | Frequency | Adverse Effect |
Infections and Infestations | Unknown | increased risk of infections, candida fungal infection, worsening of eye infections |
Blood and Lymphatic System Disorders | Unknown | increased white blood cells |
Immune System Disorders | Unknown | allergic reactions |
Endocrine Disorders | Unknown | withdrawal syndrome (headache, nausea, dizziness, decreased appetite, weakness, mood changes, altered level of consciousness, and inappropriate reactions to stress situations), growth retardation in children, Cushing's syndrome mainly characterized by a change in fat distribution (moon face, central obesity, buffalo hump), thyroid disorder, manifestation of latent hyperparathyroidism |
Metabolic and Nutrition Disorders | Unknown | increased appetite, decreased potassium and sodium levels, increased ketone bodies in blood, hyperglycemic hyperosmolar state, tumor lysis syndrome, altered acid-base balance in the body |
Psychiatric Disorders | Unknown | euphoria, depression, corticosteroid-induced psychotic disorder, insomnia, mood changes, personality changes, mania |
Nervous System Disorders | Unknown | fat deposition around the spinal cord, increased intracranial pressure in children with papilledema (more frequent when withdrawing the medication), worsening of epilepsy, after intravenous administration, a tingling sensation may appear in some areas of the body that usually disappears within a few minutes |
Eye Disorders | Unknown | cataract, glaucoma, papilledema, posterior subcapsular cataracts, corneal or scleral thinning, central serous chorioretinopathy, blurred vision |
Cardiac Disorders | Unknown | worsening of congestive heart failure, myocardial disorder, arrhythmia, thickening of the heart muscle (hypertrophic cardiomyopathy) in premature newborns |
Vascular Disorders | Unknown | thromboembolism, hypertension, thrombosis |
Gastrointestinal Disorders | Unknown | feeling of heaviness after meals, gastric or duodenal ulcer, pancreatitis, gastric mucosa inflammation, pain in the upper abdomen, abdominal discomfort, nausea |
Skin and Subcutaneous Tissue Disorders | Unknown | skin atrophy, bruising, increased hair growth, stretch marks, acne, dermatitis, increased sweating, peeling in an extensive area of the skin greater than 30% of the body surface (toxic epidermal necrolysis), widespread rash with blisters and skin peeling that occurs especially around the mouth, nose, eyes, and genitals (Stevens-Johnson syndrome), redness and itching |
Musculoskeletal and Connective Tissue Disorders | Unknown | osteoporosis, bone fractures, muscle disorders, bone death, tendon rupture and inflammation |
Renal and Urinary Disorders | Unknown | need to urinate at night, kidney stones |
Reproductive System and Breast Disorders | Unknown | menstrual irregularities, including absence |
General Disorders and Administration Site Conditions | Unknown | healing alterations |
Investigations | Unknown | weight gain, decreased blood cells (eosinophils and lymphocytes), increased blood cells (platelets), elevated cholesterol in blood, elevated triglycerides in blood, increased lipoproteins, increased intraocular pressure, alterations in diabetes control |
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor, even if it is a possible adverse effect that does not appear in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaRAM.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Actocortina
Keep out of sight and reach of children.
Do not use this medicine after the expiration date that appears on the label and carton. The expiration date is the last day of the month indicated.
Store below 25°C. Store in the original packaging to protect from light.
Once the solution is reconstituted, it should be stored in the refrigerator (between 2°C and 8°C) and cannot be used after 24 hours from reconstitution.
Medicines should not be thrown away through wastewater or household waste. Deposit the packaging and medicines you no longer need at the SIGRE Point of the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Composition of Actocortina
- The active ingredient is sodium hydrocortisone phosphate. Each vial contains 74.53 mg of hydrocortisone (equivalent to 100 mg of sodium hydrocortisone phosphate).
- The other component is water for injection.
Appearance of Actocortina and Package Contents
White to bone-colored powder.
The sterile powder is packaged in a 5 ml type I colorless glass vial.
The clear and colorless solvent is packaged in ampoules.
Each package contains 10 vials with sodium hydrocortisone phosphate powder and 10 ampoules of solvent.
Marketing Authorization Holder
CHEPLAPHARM Arzneimittel GmbH
Ziegelhof 24
17489 Greifswald
Germany
Manufacturer
B.BRAUN MEDICAL, S.A.
Ronda de los Olivares
Polígono Industrial Los Olivares
Parcela 11. 23009; Jaén
Spain
Local Representative
Laboratorios Rubió, S.A.
Industria, 29 - Pol. Ind. Comte de Sert
08755 Castellbisbal (Barcelona)
Spain
Date of the Last Revision of this Prospectus:August 2025
Other Sources of Information
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Dissolve the contents of the vial with water for injection and administer by intramuscular injection, slow intravenous injection, drop by drop, or continuous infusion:
- Actocortina 75 mg with 1 ml of water for injection
In the absence of compatibility studies, this medicine should not be mixed with others.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ACTOCORTINA 75 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 373 mgActive substance: hydrocortisoneManufacturer: Cheplapharm Arzneimittel GmbhPrescription requiredDosage form: CAPSULE, 0.5 mgActive substance: hydrocortisoneManufacturer: Diurnal Europe B.V.Prescription requiredDosage form: CAPSULE, 1 mgActive substance: hydrocortisoneManufacturer: Diurnal Europe B.V.Prescription required
Online doctors for ACTOCORTINA 75 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about ACTOCORTINA 75 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











