ABRYSVO powder and solvent for injectable solution

How to use ABRYSVO powder and solvent for injectable solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Abrysvo Powder and Solvent for Solution for Injection
vaccine against respiratory syncytial virus (bivalent, recombinant)
This medicine is subject to additional monitoring, which will allow for the rapid detection of new safety information. You can help by reporting any side effects you may have. The last section of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you start receiving this vaccine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Abrysvo and what is it used for
- What you need to know before you receive Abrysvo
- How Abrysvo is administered
- Possible side effects
- Storage of Abrysvo
- Contents of the pack and further information
1. What is Abrysvo and what is it used for
Abrysvo is a vaccine to prevent lung disease (of the respiratory tract) caused by a virus called respiratory syncytial virus (RSV). Abrysvo is administered to:
- pregnant women to protect their babies from birth up to 6 months of age,
or
- people aged 18 years and older.
RSV is a common virus that, in most cases, causes mild symptoms similar to those of a cold, such as sore throat, cough, or runny nose. However, in small infants, RSV can cause serious lung problems. In older adults and people with chronic conditions, RSV can worsen diseases such as chronic obstructive pulmonary disease (COPD) and congestive heart failure (CHF). RSV can lead to hospitalization in severe cases and, in some circumstances, can be fatal.
How Abrysvo works
This vaccine helps the immune system (the body's natural defenses) to produce antibodies (substances in the blood that help the body fight infections) that protect against lung disease caused by RSV. In pregnant women vaccinated between 24 and 36 weeks of pregnancy, these antibodies pass to the baby through the placenta before birth, protecting the babies when they are most at risk of contracting RSV.
2. What you need to know before you start receiving Abrysvo
Abrysvo must not be administered
- if you are allergic to the active substances or to any of the other components of this vaccine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you are given this vaccine
If any of the above situations apply to you (or you are not sure), talk to your doctor, pharmacist, or nurse before you are given Abrysvo.
As with any vaccine, it is possible that Abrysvo may not completely protect everyone who receives it.
Children and adolescents
Abrysvo is not recommended in children and young people under 18 years of age, except during pregnancy (see section "Pregnancy" below).
Other medicines and Abrysvo
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicine or have recently received any other vaccine.
Abrysvo can be administered at the same time as the flu vaccine or the COVID-19 vaccine. It is recommended to leave an interval of at least two weeks between the administration of Abrysvo and the tetanus, diphtheria, and pertussis vaccine.
Pregnancy and breastfeeding
Pregnant women can receive this vaccine at the end of the second or third trimester (weeks 24 to 36). If you are breastfeeding, talk to your doctor or nurse before receiving this vaccine.
Driving and using machines
It is unlikely that Abrysvo will affect your ability to drive or use machines.
Abrysvo contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; i.e., it is essentially "sodium-free".
Abrysvo contains polysorbate 80
A dose of Abrysvo contains 0.08 mg of polysorbate 80. Polysorbates can cause allergic reactions. Tell your doctor if you have any known allergy.
3. How Abrysvo is administered
You will be given an injection of 0.5 ml into a muscle in the upper arm.
If you have any questions about the use of Abrysvo, talk to your doctor, pharmacist, or nurse.
4. Possible side effects
Like all vaccines, this vaccine can cause side effects, although not everybody gets them.
Serious side effects
Very rare(may affect up to 1 in 10,000 people)
- severe allergic reactions: the signs of a severe allergic reaction include swelling of the face, lips, tongue, or throat, difficulty breathing or swallowing, and dizziness. See also section 2.
- Guillain-Barré syndrome (a neurological disorder that usually starts with tingling and weakness in the limbs and can progress to paralysis of part or all of the body).
Tell your doctor immediately if you notice signs of these serious side effects.
The following side effects have been reported in pregnant women
Very common(may affect more than 1 in 10 people)
- pain at the injection site
- headache
- muscle pain (myalgia).
Common(may affect up to 1 in 10 people)
- redness at the injection site
- swelling at the injection site.
Rare(may affect up to 1 in 1,000 people)
- allergic reactions such as rash or hives
- inflammation of the glands (lymphadenopathy).
No side effects have been reported in infants born to vaccinated mothers.
The following side effects have been reported in people aged 18 years and older
Very common(may affect more than 1 in 10 people)
- fatigue
- headache
- pain at the injection site
- muscle pain (myalgia).
Common(may affect up to 1 in 10 people)
- joint pain (arthralgia)
- redness at the injection site
- swelling at the injection site.
Uncommon(may affect up to 1 in 100 people)
- fever (pyrexia).
Rare(may affect up to 1 in 1,000 people)
- allergic reactions such as rash or hives
- inflammation of the glands (lymphadenopathy)
- bruising at the injection site (hematoma)
- itching at the injection site (pruritus).
Very rare(may affect up to 1 in 10,000 people)
- severe allergic reactions (see Serious side effects above)
- Guillain-Barré syndrome (see Serious side effects above).
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Abrysvo
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and label after EXP. The expiry date is the last day of the month shown.
Store in a refrigerator (between 2°C and 8°C).
Do not freeze. Discard if the pack has been frozen.
After reconstitution, Abrysvo should be administered immediately or within 4 hours if stored between 15°C and 30°C. Do not freeze.
6. Container Contents and Additional Information
Composition of Abrysvo
The active ingredients are:
Stabilized F antigen in pre-fusion of subgroup A of VRS1,2 60 micrograms
Stabilized F antigen in pre-fusion of subgroup B of VRS1,2 60 micrograms
(VRS antigens)
1stabilized F glycoprotein in the pre-fusion conformation.
2produced in Chinese hamster ovary (CHO) cells using recombinant DNA technology.
The other components are:
Powder
- tromethamine
- hydrochloride tromethamine
- sucrose
- mannitol (E421)
- polysorbate 80 (E433)
- sodium chloride
- hydrochloric acid
Solvent
- water for injectable preparations
Appearance of the Product and Container Contents
Abrysvo is provided as:
- white powder in a glass vial
- solvent in a pre-filled syringe or a vial for dissolving the powder.
After dissolving the powder in the solvent, the solution is transparent and colorless.
Abrysvo is available in:
- a pack with 1 vial of powder, 1 pre-filled syringe of solvent, 1 vial adapter with 1 needle or without needles (1-dose pack);
- a pack with 5 vials of powder, 5 pre-filled syringes of solvent, 5 vial adapters with 5 needles or without needles (5-dose pack);
- a pack with 10 vials of powder, 10 pre-filled syringes of solvent, 10 vial adapters with 10 needles or without needles (10-dose pack);
- a pack with 5 vials of powder and 5 vials of solvent (5-dose pack).
- a pack with 10 vials of powder and 10 vials of solvent (10-dose pack).
Only some pack sizes may be marketed.
Marketing Authorization Holder
Pfizer Europe MA EEIG
Boulevard de la Plaine 17
1050 Brussels
Belgium
Manufacturer
Pfizer Manufacturing Belgium NV
Rijksweg 12
2870 Puurs-Sint-Amands
Belgium
Pfizer Ireland Pharmaceuticals
Grange Castle Business Park
Clondalkin, Dublin 22
Ireland
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Luxembourg/Luxemburg Pfizer NV/SA Tel: + 32 (0)2 554 62 11 | Latvija Pfizer Luxembourg SARL filiale Latvija Tel.: + 371 670 35 775 |
| Lietuva Pfizer Luxembourg SARL filialas Lietuvoje Tel: +370 5 251 4000 |
Ceská republika Pfizer, spol. s r.o. Tel: +420 283 004 111 | Magyarország Pfizer Kft Tel: + 36 1 488 37 00 |
Danmark Pfizer ApS Tlf: + 45 44 20 11 00 | Malta Vivian Corporation Ltd. Tel: + 356 21344610 |
Deutschland PFIZER PHARMA GmbH Tel: +49 (0)30 550055-51000 | Nederland Pfizer bv Tel: +31 (0)800 63 34 636 |
Eesti Pfizer Luxembourg SARL Eesti filiaal Tel: +372 666 7500 | Norge Pfizer AS Tlf: +47 67 52 61 00 |
Ελλάδα Pfizer Ελλάς A.E. Τηλ.: +30 210 6785800 | Österreich Pfizer Corporation Austria Ges.m.b.H Tel: +43 (0)1 521 15-0 |
España Pfizer, S.L. Tel: +34 91 490 99 00 | Polska Pfizer Polska Sp. z o.o. Tel.: +48 22 335 61 00 |
France Pfizer Tél +33 (0)1 58 07 34 40 | Portugal Laboratórios Pfizer, Lda. Tel: +351 21 423 5500 |
Hrvatska Pfizer Croatia d.o.o. Tel: + 385 1 3908 777 | România Pfizer Romania S.R.L Tel: +40 (0) 21 207 28 00 |
Ireland Pfizer Healthcare Ireland Unlimited Company Tel: +1800 633 363 (toll free) Tel: +44 (0)1304 616161 | Slovenija Pfizer Luxembourg SARL Pfizer, podružnica za svetovanje s podrocja farmacevtske dejavnosti, Ljubljana Tel.: +386 (0)1 52 11 400 |
Ísland Icepharma hf. Simi: + 354 540 8000 | Slovenská republika Pfizer Luxembourg SARL, organizacná zložka Tel: + 421 2 3355 5500 |
Italia Pfizer S.r.l. Tel: +39 06 33 18 21 | Suomi/Finland Pfizer Oy Puh/Tel: +358 (0)9 430 040 |
Κύπρος Pfizer Ελλάς Α.Ε. (Cyprus Branch) Τηλ: +357 22817690 | Sverige Pfizer AB Tel: +46 (0)8 550 520 00 |
Date of Last Revision of this Leaflet:04/2025.
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: https://www.ema.europa.eu
--------------------------------------------------------------------------------------------------------------
This information is intended for healthcare professionals only:
Traceability
In order to improve the traceability of biological medicinal products, the name and batch number of the administered product must be clearly recorded.
Administration
Abrysvo can only be administered via the intramuscular route.
The unopened vial is stable for 5 days when stored at temperatures between 8 °C and 30 °C. At the end of this period, Abrysvo must be used or discarded. This information is used to guide healthcare professionals only in case of temporary deviations from the temperature.
Storage of the Reconstituted Vaccine
Abrysvo should be administered immediately after reconstitution or within 4 hours. Store the reconstituted vaccine between 15 °C and 30 °C. Do not freeze the reconstituted vaccine.
Chemical and physical stability has been demonstrated for 4 hours between 15 °C and 30 °C. From a microbiological point of view, the vaccine should be used immediately. If not used immediately, the storage times and conditions before use are the responsibility of the user.
Preparation for Administration
For use with the Abrysvo antigen vial (powder), pre-filled syringe with solvent, and vial adapter
The powder must be reconstituted only with the solvent provided in the pre-filled syringe using the vial adapter.
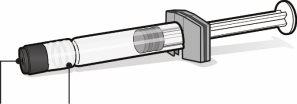
Pre-filled Syringe with Solvent for Abrysvo | Vial with Abrysvo Antigens (Powder) | Vial Adapter | |
|
|
| |
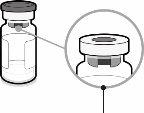
Syringe Cap | Luer Lock Adapter | Vial Stopper (without the removable closure cap) |
| Step1. Place the Vial Adapter
|
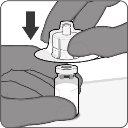
| Step2. Reconstitute the Powder Component (Antigens) to Form Abrysvo
|
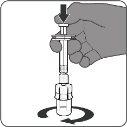
| Step3. Withdraw the Reconstituted Vaccine
|
The prepared vaccine is a clear and colorless solution. Visually inspect the vaccine for particulate matter and color changes before administration. Do not use if you observe particulate matter or color changes.
For use with the Abrysvo antigen vial (powder) and the solvent vial
The powder must be reconstituted only with the solvent vial provided.
- Using a sterile needle and syringe, withdraw all the contents of the solvent vial and inject all the contents of the syringe into the powder vial.
- Gently shake the vial with circular movements until the powder is completely dissolved. Do not shake.
- Withdraw 0.5 ml from the vial with the reconstituted vaccine.
The prepared vaccine is a clear and colorless solution. Visually inspect the vaccine for particulate matter and color changes before administration. Do not use if you observe particulate matter or color changes.
Disposal
Disposal of the unused product and all materials that have come into contact with it will be carried out in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ABRYSVO powder and solvent for injectable solutionDosage form: INJECTABLE, 0.5 mLActive substance: respiratory syncytial virus vaccinesManufacturer: Glaxosmithkline BiologicalsPrescription requiredDosage form: INJECTABLE, 50 µgActive substance: respiratory syncytial virus vaccinesManufacturer: Moderna Biotech Spain S.L.Prescription requiredDosage form: INJECTABLE, 0.5 mlActive substance: ebola vaccinesManufacturer: Janssen-Cilag International N.VPrescription required
Online doctors for ABRYSVO powder and solvent for injectable solution
Discuss questions about ABRYSVO powder and solvent for injectable solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions