SANDOSTATIN LAR 20 mg POWDER AND SOLVENT FOR SUSPENSION FOR INJECTION

How to use SANDOSTATIN LAR 20 mg POWDER AND SOLVENT FOR SUSPENSION FOR INJECTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
SANDOSTATIN LAR 20 mg powder and solvent for injectable suspension
octreotide
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Sandostatin LAR is and what it is used for
- What you need to know before you use Sandostatin LAR
- How to use Sandostatin LAR
- Possible side effects
- Storage of Sandostatin LAR
- Contents of the pack and other information
1. What Sandostatin LAR is and what it is used for
Sandostatin LAR is a synthetic compound derived from somatostatin. Somatostatin is normally found in the human body, where it inhibits the release of certain hormones such as growth hormone. The advantages of Sandostatin LAR over somatostatin are that it is more potent and its effects are longer-lasting.
Sandostatin LAR is used
- to treat acromegaly,
Acromegaly is a disease in which the body produces too much growth hormone. Normally, growth hormone controls the growth of tissues, organs, and bones. An excess of growth hormone means an increase in the size of bones and tissues, especially in the hands and feet. Sandostatin LAR significantly reduces the symptoms of acromegaly, which include headache, excessive sweating, numbness of the hands and feet, fatigue, and joint pain. In most cases, the overproduction of growth hormone is caused by an increase in the size of the pituitary gland (pituitary adenoma); treatment with Sandostatin LAR can reduce the size of the adenoma.
Sandostatin LAR is used to treat people who suffer from acromegaly:
- when other types of treatment for acromegaly (surgery or radiotherapy) are not suitable or have not worked properly;
- after radiotherapy, to cover the intermediate period until the radiotherapy is fully effective.
- to relieve symptoms associated with the overproduction of certain specific hormones and other related substances in the stomach, intestine, or pancreas.
The overproduction of certain specific hormones and other natural substances can be caused by rare disorders of the stomach, intestine, or pancreas. This disrupts the natural hormonal balance and causes a series of symptoms such as hot flashes, diarrhea, low blood pressure, skin rash, and weight loss. Treatment with Sandostatin LAR helps control these symptoms.
- to treat neuroendocrine tumors located in the intestine (e.g., appendix, small intestine, or colon)
Neuroendocrine tumors are rare tumors that can be found in different parts of the body. Sandostatin LAR is also used to control the growth of these tumors when they are located in the intestine (e.g., appendix, small intestine, or colon)
- to treat pituitary tumors that produce too much thyroid-stimulating hormone (TSH).
An excess of thyroid-stimulating hormone (TSH) causes hyperthyroidism. Sandostatin LAR is used to treat people with pituitary tumors that produce too much thyroid-stimulating hormone (TSH):
- when other types of treatment (surgery or radiotherapy) are not suitable or have not worked;
after radiotherapy, to cover the period until the radiotherapy is fully effective.
2. What you need to know before you use Sandostatin LAR
Follow all the instructions given by your doctor carefully. They may be different from the information contained in this leaflet.
Read the following instructions before using Sandostatin LAR.
Do not use Sandostatin LAR:
- if you are allergic to octreotide or any of the other components of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor before starting treatment with Sandostatin LAR:
- if you know you currently have gallstones, or have had them in the past, or have any complications such as fever, chills, abdominal pain, or yellowing of the skin or eyes; inform your doctor, as prolonged use of Sandostatin LAR may cause gallstones to form. Your doctor may want to check your gallbladder periodically.
- if you know you have diabetes, as Sandostatin LAR may affect blood sugar levels. If you are diabetic, you should regularly check your blood sugar levels.
- if you have a history of vitamin B12 deficiency, your doctor may periodically check your B12 levels.
Tests and checks
If you receive treatment with Sandostatin LAR for a prolonged period, your doctor may periodically check your thyroid function.
Your doctor will check your liver function.
Your doctor may check your pancreatic enzyme function.
Children
There is limited experience with the use of Sandostatin LAR in children.
Using Sandostatin LAR with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Normally, you can continue taking other medicines while being treated with Sandostatin LAR. However, it has been reported that some medicines such as cimetidine, cyclosporine, bromocriptine, quinidine, and terfenadine are affected by Sandostatin LAR.
If you are taking a medicine to control blood pressure (e.g., a beta-blocker or a calcium channel blocker) or an agent to control fluid and electrolyte balance, your doctor may need to adjust the dose.
If you are diabetic, your doctor may need to adjust your insulin dose.
If you are going to receive treatment with lutetium (177Lu) oxodotreotide, a radiopharmaceutical, your doctor may interrupt and/or adapt the treatment with Sandostatin LAR for a short period.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before using this medicine.
Sandostatin LAR should only be used during pregnancy if it is strictly necessary.
Women of childbearing age should use an effective contraceptive method during treatment.
You should not breastfeed while being treated with Sandostatin LAR. It is not known whether Sandostatin LAR passes into breast milk.
Driving and using machines
Sandostatin LAR has no or negligible influence on the ability to drive and use machines. However, some of the side effects you may experience during treatment with Sandostatin LAR, such as headache and fatigue, may reduce your ability to drive and use machines safely.
Sandostatin contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per vial; it is essentially "sodium-free".
3. How to use Sandostatin LAR
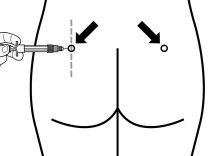
Sandostatin LAR should always be administered as an injection into the muscle of the buttocks. With repeated administration, the right and left buttocks should be used alternately.
If you use more Sandostatin LAR than you should
No life-threatening reactions have been reported after an overdose with Sandostatin LAR.
The symptoms of overdose are: hot flashes, frequent urination, fatigue, depression, anxiety, and lack of concentration.
If you think you have had an overdose and experience any of these symptoms, inform your doctor immediately. You can also call the Toxicology Information Service, Tel. 91 562 0420.
If you forget to use Sandostatin LAR
If you have missed your injection, it is recommended that you have it administered as soon as you remember, and then continue with your usual schedule. It will not harm you to receive a dose a few days late, but your symptoms may temporarily recur until you return to your usual treatment schedule.
If you stop treatment with Sandostatin LAR
If you stop your treatment with Sandostatin LAR, your symptoms may recur. Therefore, do not stop treatment with Sandostatin LAR unless your doctor tells you to.
If you have any further questions on the use of this medicine, ask your doctor, nurse, or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some side effects could be serious. Tell your doctor immediately if you experience any of the following:
Very common(may affect more than 1 in 10 people):
- Gallstones, which can cause sudden back pain.
- Too much sugar in the blood.
Common(may affect up to 1 in 10 people):
- Decreased activity of the thyroid gland (hypothyroidism) which can cause changes in heart rate, appetite, or weight; fatigue, feeling cold, or swelling in the front of the neck.
- Changes in thyroid function tests.
- Inflammation of the gallbladder (cholecystitis); symptoms may include pain in the upper right abdomen, fever, nausea, yellowing of the skin and eyes (jaundice).
- Too little sugar in the blood.
- Alteration of glucose tolerance.
- Slow heart rate.
Uncommon(may affect up to 1 in 100 people):
- Thirst, low urine output, dark urine, dry reddened skin.
- Fast heart rate.
Other serious side effects
- Hypersensitivity reactions (allergic reactions) including skin rash.
- A type of allergic reaction (anaphylaxis) that can cause difficulty swallowing or breathing, swelling, and tingling, possibly with a decrease in blood pressure with dizziness or loss of consciousness.
- Inflammation of the pancreas (pancreatitis); symptoms may include sudden pain in the upper abdomen, nausea, vomiting, diarrhea.
- Inflammation of the liver (hepatitis); symptoms may include yellowing of the skin and eyes (jaundice), nausea, vomiting, loss of appetite, general feeling of discomfort, itching, lightly colored urine.
- Irregular heartbeat.
- Low platelet count in the blood; this can increase bleeding or bruising.
Tell your doctor immediately if you notice any of the above side effects.
Other side effects:
Tell your doctor, pharmacist, or nurse if you notice any of the following side effects. They are usually mild and tend to disappear as treatment progresses.
Very common(may affect more than 1 in 10 people):
- Diarrhea.
- Abdominal pain.
- Nausea.
- Constipation.
- Flatulence (gas).
- Headache.
- Local pain at the injection site.
Common(may affect up to 1 in 10 people):
- Discomfort in the stomach after eating (dyspepsia).
- Vomiting.
- Feeling of having a full stomach.
- Fatty stools.
- Loose stools.
- Change in stool color.
- Dizziness.
- Loss of appetite.
- Changes in liver function tests.
- Hair loss.
- Difficulty breathing.
- Weakness.
If you experience any side effects, talk to your doctor, pharmacist, or nurse.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: http://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Sandostatin LAR
Keep this medicine out of the sight and reach of children.
Store in the original packaging to protect from light.
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
Sandostatin LAR can be stored below 25°C on the day of injection.
Do not store Sandostatin LAR after reconstitution (it must be used immediately).
Do not use this medicine after the expiry date which is stated on the carton after EXP. The expiry date is the last day of the month shown.
Do not use this medicine if you notice the presence of particles or a change in color.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Container Content and Additional Information
Sandostatin LAR Composition
- The active ingredient is octreotide.
A vial contains 20 mg of octreotide (as octreotide acetate).
- The other components are:
In the powder (vial): poly(DL-lactic-co-glycolic acid), mannitol (E421).
In the solvent (pre-filled syringe): sodium carboxymethylcellulose, mannitol (E421), poloxamer 188, water for injectable preparations
Appearance of Sandostatin LAR and Container Content
Unitary containers containing a 6 ml glass vial with a rubber stopper (bromobutyl), sealed with an aluminum flip-off cap, containing the powder for injectable suspension and a 3 ml pre-filled glass syringe with a front stopper and a plunger stopper (chlorobutyl rubber) with 2 ml of solvent, packaged together in a sealed blister tray with a vial adapter and a safety injection needle.
Multiples of three unitary containers, each containing: a 6 ml glass vial with a rubber stopper (bromobutyl), sealed with an aluminum flip-off cap, containing the powder for injectable suspension and a 3 ml pre-filled glass syringe with a front stopper and a plunger stopper (chlorobutyl rubber) with 2 ml of solvent, packaged together in a sealed blister tray with a vial adapter and a safety injection needle.
Only some pack sizes may be marketed.
Marketing Authorization Holder
Novartis Farmacéutica, S.A.
Gran Vía de les Corts Catalanes, 764
08013 Barcelona
Manufacturer
Novartis Farmacéutica, S.A.
Gran Vía de les Corts Catalanes, 764
08013 Barcelona
Novartis Pharma GmbH
Jakov-Lind-Straße 5, Top 3.05 1020 Wien Austria
Novartis Pharma NV
Medialaan 40/Bus 1, 1800 Vilvoorde, (Belgium).
Novartis Healthcare A/S
Edvard Thomsens vej 14, DK-2300 Copenhagen S, (Denmark).
Novartis Finland Oy
Metsänneidonkuja 10, 02130 Espoo, (Finland)
Novartis Pharma SAS
8-10 rue Henri Sainte-Claire Deville, 92500 Rueil-Malmaison (France)
Novartis Pharma GmbH
Roonstrasse 25, 90429 Nürnberg, (Germany).
Novartis Pharma GmbH
Sophie-Germain-Strasse 10, 90443 Nürnberg (Germany).
Novartis Farma-Produtos Farmacéuticos, SA
Avenida Professor Doutor Cavaco Silva nº10E, Taguspark, 2740-255 Porto Salvo, (Portugal).
Novartis Farma S.p.A.
Via Provinciale Schito 131, 80058 Torre Annunziata, NA , (Italy).
Novartis Farma S.p.A.
Viale Luigi Sturzo 43, 20154 - Milan (MI) (Italy)
Novartis Sverige AB
Torshamnsgatan 48, 164 40 Kista (Sweden)
Novartis (Hellas) S.A.
12th km National Road Athinon-Lamia 14451 Metamorphosis Attiki, (Greece).
Novartis Pharma B.V.
Haaksbergweg 16, 1101 BX Amsterdam (Netherlands).
Novartis Poland Sp. z o.o.
15 Marynarska Street, 02-674 Warsaw (Poland).
Novartis Hungária Kft.
Vasút u.13, 2040 Budaörs (Hungary).
Abbot Biologicals B.V.
Veerweg, 12. 8121 AA Olst (Netherlands)
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, Germany, Greece, Hungary, Iceland, Ireland, Latvia, Lithuania, Malta, Norway, Poland, Romania, Slovakia, Slovenia, Spain, Sweden | Sandostatin LAR |
Belgium, Luxembourg, Netherlands | Sandostatine LAR |
Italy, Portugal | Sandostatina LAR |
France | Sandostatine L.P. |
Date of the last revision of this leaflet:12/2023
Other sources of information
Detailed and up-to-date information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
This information is intended only for healthcare professionals:
How much Sandostatin LAR should be used
Acromegaly
Treatment should be initiated with the administration of 20 mg of Sandostatin LAR at intervals of 4 weeks for 3 months. Patients being treated with Sandostatin s.c. may initiate treatment with Sandostatin LAR the day after the last dose of Sandostatin s.c.. Subsequent dose adjustment should be based on serum growth hormone (GH) and insulin-like growth factor 1 (IGF-1) concentrations and clinical symptoms.
For patients in whom clinical symptoms and biochemical parameters (GH; IGF-1) are not fully controlled after a period of 3 months (GH concentrations still above 2.5 micrograms/L), the dose may be increased to 30 mg every 4 weeks. If, after 3 months, GH, IGF-1, and/or symptoms are not adequately controlled at the 30 mg dose, the dose may be increased to 40 mg every 4 weeks.
For patients with GH concentrations consistently below 1 microgram/L, and with normalized serum IGF-1 concentrations, and in whom most reversible signs/symptoms of acromegaly have disappeared after 3 months of treatment with 20 mg, 10 mg of Sandostatin LAR may be administered every 4 weeks. However, especially in this group of patients, it is recommended to closely monitor serum GH and IGF-1 concentrations, and signs/symptoms at this low dose of Sandostatin LAR.
For patients being treated with a stable dose of Sandostatin LAR, GH and IGF-1 assessment should be performed every 6 months.
Gastroenteropancreatic neuroendocrine tumors
- Treatment of patients with symptoms associated with functional gastroenteropancreatic neuroendocrine tumors
Treatment should be initiated with the administration of 20 mg of Sandostatin LAR at intervals of 4 weeks. Patients being treated with Sandostatin s.c. should continue at the previously effective dose for 2 weeks after the first injection of Sandostatin LAR.
For patients in whom symptoms and biochemical markers are well controlled after 3 months of treatment, the dose may be reduced to 10 mg of Sandostatin LAR every 4 weeks.
For patients in whom symptoms are only partially controlled after 3 months of treatment, the dose may be increased to 30 mg of Sandostatin LAR every 4 weeks.
For days when symptoms associated with gastroenteropancreatic tumors may increase during treatment with Sandostatin LAR, additional administration of Sandostatin s.c. at the dose used before treatment with Sandostatin LAR is recommended. This may occur mainly in the first 2 months of treatment until therapeutic concentrations of octreotide are reached.
- Treatment of patients with advanced neuroendocrine tumors of the intestine or unknown origin where the places of origin that are not of intestinal origin have been excluded
The recommended dose of Sandostatin LAR is 30 mg administered every 4 weeks. Treatment with Sandostatin LAR to control the tumor should be continued in the absence of tumor progression.
Treatment of thyrotropin-secreting adenomas
Treatment with Sandostatin LAR should be initiated at a dose of 20 mg at intervals of 4 weeks for 3 months before considering a dose adjustment. Afterward, the dose is adjusted based on TSH and thyroid hormone response.
Instructions for preparation and intramuscular injection of Sandostatin LAR.
FOR INTRAMUSCULAR INJECTION ONLY
Components of the injection kit:
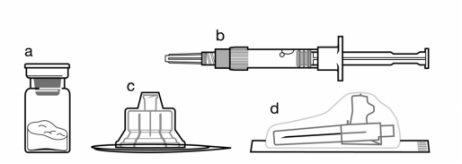
- A vial containing the Sandostatin LAR powder
- A pre-filled syringe containing the solvent for reconstitution
- A vial adapter for reconstitution of the medicinal product
- A safety injection needle
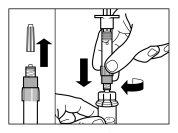
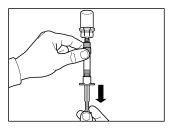
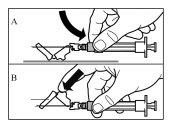
Follow the instructions below carefully to ensure the reconstitution of Sandostatin LAR before intramuscular injection
There are three critical steps in the Sandostatin LAR reconstitution process. If these are not performed correctly, it may result in inadequate administration of the medicinal product.
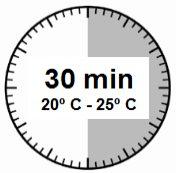
- The injection kit must reach room temperature. Remove the injection kit from the refrigerator and let it reach room temperature for at least 30 minutes before reconstitution, but not exceeding 24 hours.
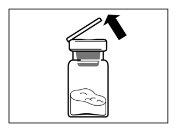
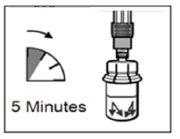
- After adding the diluent solution, let the vial stand for 5 minutes to ensure that the powder is completely saturated.
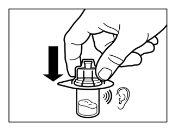
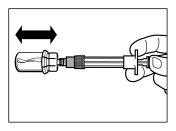
- After saturation, moderately shake the vialin a horizontal direction for at least 30 seconds until a uniform suspension is formed. The Sandostatin LAR suspension should only be prepared immediatelybefore administration.
Sandostatin LAR must be administered only by an expert healthcare professional.
| STEP 1
WARNING: it is essential to start the reconstitution process only after the injection kit has reached room temperature. Let the kit reach room temperature for at least 30 minutes before reconstitution, but not exceeding 24 hours. Note: The injection kit can be refrigerated again if necessary. |
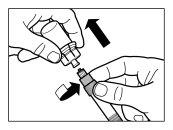
| STEP 2
|
| STEP 3
|
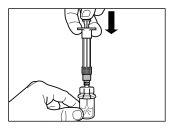
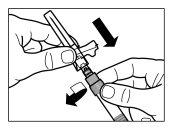
| |
| STEP 4 WARNING: it is essential to let the vial stand for 5 minutesto ensure that the solvent has completely saturated the powder. Note: it is normal for the plunger to move upwards as there may be overpressure in the vial.
|
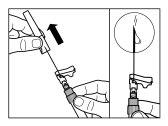
| STEP 5
WARNING:Keep the plunger pressed and moderately shake the vialin a horizontal direction for at least 30 secondsto ensure that the powder is completely suspended in the solvent (uniform suspension of a milky consistency). Repeat moderate shaking for another 30 seconds if the powder is not completely suspended. |
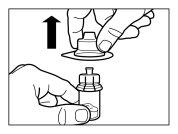
| STEP 6
|
| |
| STEP 7
|
| STEP 8
|
| STEP 9
|
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SANDOSTATIN LAR 20 mg POWDER AND SOLVENT FOR SUSPENSION FOR INJECTIONDosage form: INJECTABLE, Octreotide 100 micrograms/mlActive substance: octreotideManufacturer: Gp Pharm S.A.Prescription requiredDosage form: INJECTABLE, Octreotide 500 micrograms/mlActive substance: octreotideManufacturer: Gp Pharm S.A.Prescription requiredDosage form: INJECTABLE, 200 micrograms/mlActive substance: octreotideManufacturer: Gp Pharm S.A.Prescription required
Online doctors for SANDOSTATIN LAR 20 mg POWDER AND SOLVENT FOR SUSPENSION FOR INJECTION
Discuss questions about SANDOSTATIN LAR 20 mg POWDER AND SOLVENT FOR SUSPENSION FOR INJECTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions