
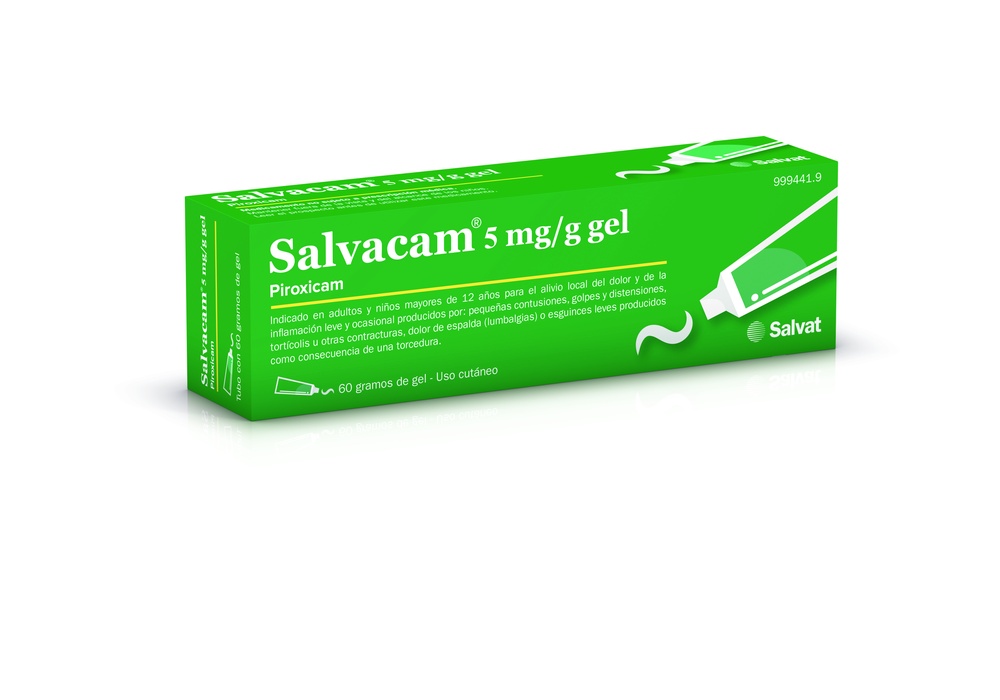
SALVACAM 5mg/g GEL

How to use SALVACAM 5mg/g GEL
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Salvacam 5 mg/g Gel
Piroxicam
Read the entire package leaflet carefully before starting to use this medication, as it contains important information for you.
Follow the administration instructions for the medication contained in this package leaflet or as indicated by your doctor or pharmacist.
- Keep this package leaflet, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this package leaflet. See section 4.
- You should consult a doctor if it worsens or does not improve after 7 days.
Contents of the Package Leaflet
- What Salvacam is and what it is used for
- What you need to know before starting to use Salvacam
- How to use Salvacam
- Possible side effects
- Storage of Salvacam
- Package Contents and Additional Information
1. What Salvacam is and what it is used for
Piroxicam, the active ingredient of this medication, belongs to the group of non-steroidal anti-inflammatory drugs and acts by reducing pain.
Salvacam is indicated in adults and children over 12 years of age for the local relief of pain and mild, occasional inflammation caused by: minor bruises, bumps, and sprains, torticollis, or other contractures, back pain (lumbago) or mild sprains resulting from a twist.
You should consult a doctor if it worsens or does not improve after 7 days.
2. What you need to know before starting to use Salvacam
Do not use Salvacam:
- if you are allergic to piroxicam or any of the other components of this medication (listed in section 6).
- if you have ever suffered an allergic reaction (hypersensitivity) after taking acetylsalicylic acid or any other non-steroidal anti-inflammatory drug (NSAIDs).
- if acetylsalicylic acid or other non-steroidal anti-inflammatory medications cause you symptoms such as rhinitis, asthma, swelling of the face, lips, mouth, tongue, or throat that can cause difficulty swallowing or breathing (angioedema) or skin rash.
- if you are in the last 3 months of pregnancy.
Warnings and Precautions
Consult your doctor or pharmacist before starting to use Salvacam.
- Salvacam should not come into contact with the eyes or mucous membranes, nor should it be applied to open skin lesions.
- Do not use Salvacam under occlusive dressing; the treated area must be uncovered and in contact with the air.
- If the use of Salvacam causes irritation in the application area, consult your doctor.
- If you experience a skin rash or skin symptoms, stop using piroxicam immediately, seek urgent medical advice, and inform your doctor that you are using this medication.
- If you experience symptoms or signs of Stevens-Johnson syndrome or Toxic Epidermal Necrolysis (e.g., circular red spots with blisters or mucosal lesions), treatment with Salvacam gel should be discontinued, and you should immediately consult a doctor, informing them that you are taking this medication.
- With the administration of piroxicam by mouth, skin rashes that can threaten the patient's life (Stevens-Johnson syndrome and toxic epidermal necrolysis) have been described, initially appearing as red spots or circular patches, often with a central blister. These reactions have not been related to the use of piroxicam by topical route, but it cannot be entirely ruled out that they may occur with this medication.
Other additional signs that may appear are sores in the mouth, throat, nose, genitals, and conjunctivitis (swollen and red eyes).
These life-threatening skin rashes are often accompanied by flu-like symptoms. The rash can progress to the formation of generalized blisters or skin peeling.
The period of highest risk for the appearance of severe skin reactions is during the first few weeks of treatment.
- If you have developed Stevens-Johnson syndrome or toxic epidermal necrolysis with the use of piroxicam by mouth, you should not use Salvacam gel at any time.
- When the absorption of the gel is not complete, mild and transient skin discoloration has been observed.
- The treated areas should not be exposed to the sun (even on cloudy days) or to ultraviolet lamps (UVA). If the area treated with this medication is to be exposed to the sun, photosensitivity reactions may occur.
- Do not use in children under 12 years of age.
Other Medications and Salvacam
Inform your doctor or pharmacist that you are using, have recently used, or may need to use any other medication.
Do not use other topical preparations on the same application site as Salvacam without consulting your doctor or pharmacist first.
Pregnancy, Breastfeeding, and Fertility
Oral formulations (e.g., tablets) of piroxicam may have adverse effects on the fetus. It is unknown if the same risk applies to Salvacam.
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication. Do not use Salvacam if you are in the last 3 months of pregnancy. You should not use Salvacam during the first 6 months of pregnancy unless it is clearly necessary and as indicated by your doctor. If you need treatment during this period, the lowest dose should be used for the shortest possible time.
Driving and Using Machines
The influence of Salvacam on the ability to drive and use machines is nil or insignificant.
Salvacam contains Propylene Glycol
This medication contains 100 mg of propylene glycol in each gram of Salvacam gel.
3. How to use Salvacam
Follow the administration instructions for this medication as indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
Salvacam is a topical medication (for use exclusively on the skin).
Do not use occlusive dressings; the application area must be in contact with the air.
The recommended dose is:
Adults and children over 12 years of age
The dose will depend on the extent of the affected area. The normal dose is 1 gram to 3 grams of gel, equivalent to 5 and 15 mg of piroxicam:
- 1 gram of Salvacam is approximately equivalent to 3 cm of gel
- 3 grams of Salvacam are approximately equivalent to 9 cm of gel
Application will be performed 2 to 4 times a day, spreading the gel with a massage until no residue of the medication remains on the skin.
After application, wash your hands unless they are the treatment site.
Your doctor or pharmacist will indicate the duration of treatment with Salvacam.
Do not apply for more than 7 consecutive days without consulting your doctor or pharmacist.
Use in Children
This medication is not recommended for use in children under 12 years of age, as there are insufficient data available on safety and efficacy.
Use in Patients over 65 Years of Age
No dose modification is required for this group of patients.
Use in Patients with Kidney or Liver Problems
There are no special recommendations for use in these groups of patients.
Guidelines for correct preparation and administration
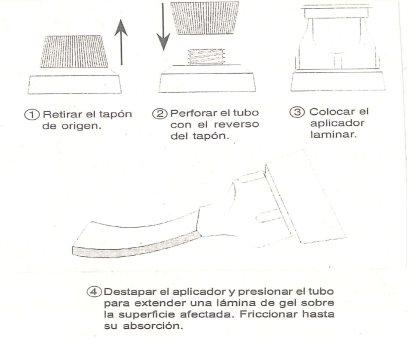
Unscrew the original cap and make an opening in the tube mouth with the top part of the same. Replace the original cap with the laminar applicator. From this moment on, the original cap can be discarded. Remove the applicator cap, spread a layer of gel over the affected surface, and gently rub until absorbed. Close the tube well after use. Wash your hands after applying the gel
If you use more Salvacam than you should
Since the application of this medication is for topical use, it is unlikely that intoxication will occur.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicological Pharmacovigilance Service. Phone 91 562 04 20, indicating the medication and the amount ingested.
If you forget to use Salvacam
Do not apply a double dose to make up for forgotten doses.
If you interrupt treatment with Salvacam
If you have any other doubts about the use of this medication, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medications, Salvacam can cause side effects, although not everyone will experience them.
- Uncommon side effects (may affect 1 to 10 in every 1,000 patients): erythema (redness), pruritus (itching).
- Rare side effects (may affect 1 to 10 in every 10,000 patients):
- photosensitivity reaction.
- Side effects of unknown frequency (cannot be estimated from available data): dermatitis, desquamation in the application area, skin irritation, and application site irritation, fixed drug eruption (may appear as rounded or oval plaques with redness and swelling of the skin), blisters (urticaria), pruritus (itching).
With the administration of piroxicam by mouth, the following serious side effects have been reported with a very rare frequency (may affect less than 1 in every 10,000 patients): life-threatening skin rashes (Stevens-Johnson syndrome and toxic epidermal necrolysis). These reactions have not been related to the use of piroxicam by topical route, but it cannot be entirely ruled out that they may occur with this medication.
When the absorption of the gel is not complete due to insufficient rubbing, mild and transient skin discoloration has been observed.
Reporting of Side Effects
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect not listed in this package leaflet.
You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medications http://www.notificaRAM.es.
By reporting side effects, you can contribute to providing more information on the safety of this medication.
.
5. Storage of Salvacam
No special storage conditions are required.
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date shown on the packaging after "Expiration Date". The expiration date is the last day of the month indicated.
Medications should not be disposed of through wastewater or household waste. Deposit the packaging and medications you no longer need at the SIGRE Point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Composition of Salvacam
The active ingredient is piroxicam. Each gram of gel contains 5 mg of piroxicam.
The other components (excipients) are: carbomer, isopropyl alcohol, glycerol (E-422), propylene glycol (E-1520), diisopropanolamine, and purified water.
Appearance of the Product and Package Contents
Salvacam is a yellowish, transparent gel.
It is presented in an aluminum tube with a screw cap and laminar applicator containing 60 grams of gel.
Marketing Authorization Holder and Manufacturer
Laboratorios Salvat, S.A.
C/ Gall 30 – 36 – 08950
Esplugues de Llobregat
Barcelona - Spain
Date of the Last Revision of this Package Leaflet:April 2024
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SALVACAM 5mg/g GELDosage form: GEL, 500 mg piroxicam / 100 gActive substance: piroxicamManufacturer: Laboratorios Veris S.A.Prescription requiredDosage form: CREAM, 1.5 g aceclofenac / 100 gActive substance: aceclofenacManufacturer: Almirall S.A.Prescription requiredDosage form: GEL, 50 mg/gActive substance: ibuprofenManufacturer: Arafarma Group S.A.Prescription not required
Online doctors for SALVACAM 5mg/g GEL
Discuss questions about SALVACAM 5mg/g GEL, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






