ROZLYTREK 50 mg FILM-COATED GRANULES IN SACHET

How to use ROZLYTREK 50 mg FILM-COATED GRANULES IN SACHET
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Rozlytrek 50 mg Film-Coated Granules
entrectinib
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start taking this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
- This leaflet has been written in a way that assumes the reader is the person taking the medicine. If you are giving this medicine to your child, please replace “you” with “your child” throughout.
Contents of the Package Leaflet
- What is Rozlytrek and what is it used for
- What you need to know before you take Rozlytrek
- How to take Rozlytrek
- Possible side effects
- Storage of Rozlytrek
- Contents of the pack and further information
- Instructions for use
1. What is Rozlytrek and what is it used for
What is Rozlytrek
Rozlytrek is a cancer medicine that contains the active substance entrectinib.
What Rozlytrek is used for
Rozlytrek is used to treat:
- adults and adolescents and children over 1 month of age with solid tumors (cancer) in various parts of the body, which are caused by a change in the neurotrophic tyrosine kinase receptor (NTRK) gene
- adults with a type of lung cancer called ‘non-small cell lung cancer’ (NSCLC), which is caused by a change in the ROS1 gene.
This medicine is used for solid tumors when:
- a test has shown that your cancer cells have a change in the genes called “NTRK” (see “How Rozlytrek works” below), and
- your cancer has spread within the affected organ or to other organs in your body or if surgery to remove the cancer is likely to cause serious complications, and
- you have not received previous treatment with medicines called ‘NTRK inhibitors’
- other treatments have not been effective or are not suitable for you.
This medicine is used for non-small cell lung cancer (NSCLC):
- it is ‘ROS1-positive’: this means that your cancer cells have a change in a gene called ‘ROS1’ (see “How Rozlytrek works” below),
- it is advanced – for example, it has spread to other parts of your body (i.e., it is metastatic), and
- you have not received previous treatment with medicines called ‘ROS1 inhibitors’.
How Rozlytrek works
Rozlytrek works by blocking the action of faulty proteins. These faulty proteins are caused by a change in the genes that produce them, called NTRK or ROS1. These faulty proteins stimulate the growth of cancer cells.
Rozlytrek may slow down or stop the growth of the cancer. It may also help reduce it.
2. What you need to know before you take Rozlytrek
Do not take Rozlytrek:
- if you are allergic to entrectinib or any of the other ingredients of this medicine (listed in section 6).
If you are not sure, talk to your doctor, pharmacist, or nurse before taking Rozlytrek.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before taking Rozlytrek if:
- you have recently had memory loss, confusion, hallucinations, changes in mental status
- you have had bone fractures or diseases that may increase the risk of bone fractures, called ‘osteoporosis’ or ‘osteopenia’
- you take medication to lower uric acid in the blood
- you have heart failure (when your heart has difficulty pumping blood to supply oxygen to the rest of your body) – signs may include cough, shortness of breath, and swelling in legs and arms.
- you have ever had heart problems or electrical conduction problems in the heart called ‘prolonged QTc interval’ – this is shown on an electrocardiogram (ECG) or by low levels of electrolytes in the blood
- you have a hereditary problem called “galactose intolerance”, “congenital lactase deficiency”, or “glucose-galactose malabsorption”
If you have any of the above conditions (or are not sure), talk to your doctor or pharmacist before taking Rozlytrek.
Other medicines and Rozlytrek
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. This is because Rozlytrek may affect the way other medicines work. Similarly, other medicines may affect the way Rozlytrek works.
In particular, talk to your doctor or pharmacist if you are taking any of the following medicines:
- for fungal infections (antifungals) – such as ketoconazole, itraconazole, voriconazole, posaconazole
- for HIV/AIDS infection – such as ritonavir or saquinavir
- for depression – such as paroxetine, fluvoxamine, or herbal medicine for depression – St. John's Wort
- to prevent seizures or fits – such as phenytoin, carbamazepine, or phenobarbital
- for tuberculosis – such as rifampicin or rifabutin
- for solid tumors and blood cancers – topotecan, lapatinib, mitoxantrone, apalutamide, or methotrexate
- for joint inflammation or autoimmune joint diseases (rheumatoid arthritis) – methotrexate
- for migraines – ergotamine
- for severe pain – fentanyl
- for mental illnesses (psychosis) or Tourette's syndrome – pimozide
- for irregular heartbeat – quinidine
- to prevent blood clots – warfarin or dabigatran etexilate
- for acid reflux (heartburn) – cisapride or omeprazole
- to lower cholesterol in the blood – atorvastatin, pravastatin, or rosuvastatin
- to suppress your body's immune system or prevent your body from rejecting a transplanted organ – sirolimus, tacrolimus, or cyclosporin
- to lower blood sugar levels – repaglinide or tolbutamide
- for high blood pressure – bosentan, felodipine, nifedipine, or verapamil
- for inflammation or nausea – dexamethasone
If you have any of the above conditions (or are not sure), talk to your doctor or pharmacist before taking Rozlytrek.
Using Rozlytrek with food or drinks
Do not drink grapefruit juice or eat grapefruit or bitter oranges during treatment with Rozlytrek. This may increase the amount of the medicine in your blood to harmful levels.
Pregnancy, breastfeeding, and fertility
Women and contraceptive measures
You must avoid becoming pregnant while taking this medicine, because it may harm your unborn baby. If you can become pregnant, you must use a highly effective method of contraception:
- during treatment, and
- for at least 5 weeks after stopping treatment.
Rozlytrek may reduce the effect of contraceptive medicines (birth control pills or hormonal contraceptives). You should use another reliable method of contraception such as a barrier method (e.g., condom).
Talk to your doctor about the contraceptive methods that are suitable for you and your partner.
Men and contraceptive measures
Your partner must avoid becoming pregnant while you are taking this medicine, because it may harm your unborn baby. If your partner can become pregnant, you must use a highly effective method of contraception:
- during treatment, and
- for at least 3 months after stopping treatment.
Talk to your doctor about the contraceptive methods that are suitable for you and your partner.
Pregnancy
- Do not take Rozlytrek if you are pregnant, because it may harm your unborn baby.
- If you become pregnant while taking this medicine or in the 5 weeks following your last dose, tell your doctor immediately.
Breastfeeding
Do not breastfeed while taking this medicine. It is not known whether Rozlytrek passes into breast milk and may harm your baby.
Driving and using machines
Rozlytrek may affect your ability to drive, ride a bike, or use machines. The following side effects may occur while taking Rozlytrek:
- blurred vision
- feeling tired, dizzy, or faint
- changes in mental status, confusion, or seeing things that are not there (hallucinations).
If this happens, do not drive, ride a bike, or use heavy machinery until you feel better. Ask your doctor or pharmacist if you can drive, ride a bike, or use machines.
Rozlytrek contains sodium:
This medicine contains less than 1 mmol of sodium (23 mg) per 600 mg dose; this is essentially 'sodium-free'. See section 6.
3. How to take Rozlytrek
Follow exactly the instructions for administration of this medicine given by your doctor or pharmacist. If you are not sure, talk to your doctor or pharmacist again.
How much to take
Adults:
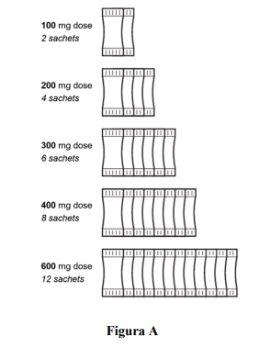
- The recommended dose is 12 sachets once a day (total amount 600 mg). Each individual sachet contains 50 mg.
- If you feel unwell, your doctor may reduce your dose, interrupt your treatment for a short period, or stop treatment completely.
Adolescents and children over 1 month of age:
- Your doctor will calculate the correct dose to be taken – based on the child's height and weight.
- Your doctor will review your child's dose and change it as necessary.
Rozlytrek is also available in the form of hard capsules for patients who can swallow whole capsules. The capsules can also be prepared as an oral suspension for patients who cannot swallow soft foods or need a feeding tube.
How to take it
Take Rozlytrek film-coated granules by mouth – sprinkling on soft foods.
- Do notdivide the contents of a sachet of film-coated granules to prepare a smaller dose.
- The film-coated granules should be sprinkled onto one or more tablespoons of a soft food (such as apple sauce, yogurt, or pudding) and taken within 20 minutes of mixing.
- Do notcrush or chew the film-coated granules to avoid the bitter taste.
- Drink water after taking the medicine.
- The film-coated granules must not be used with a feeding tube, as it may block the tube.
Read the ‘Instructions for Use’ at the end of the package leaflet.
Read and follow carefully the ‘Instructions for Use’ at the end of the package leaflet on how to take and administer Rozlytrek. Detailed information is shown on how to prepare a dose using the film-coated granules and soft foods.
If you vomit after taking Rozlytrek
If you vomit immediately after taking the dose, talk to your doctor or pharmacist about what to do next.
If you take more Rozlytrek than you should
If you take more Rozlytrek than you should, talk to a doctor or go to a hospital immediately. Take the medicine pack and this leaflet with you.
If you forget to take Rozlytrek
- If it is more than 12 hours until your next dose, take the missed dose as soon as you remember.
- If it is less than 12 hours until your next dose, do not take the missed dose. Take your next dose at the usual time.
- Do not take a double dose to make up for a missed dose.
If you stop taking Rozlytrek
Do not stop taking this medicine without talking to your doctor first. It is important that you take Rozlytrek every day for the time your doctor has prescribed.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The following side effects may occur with this medicine.
Serious side effects
Tell your doctor immediately if you experience any of the following serious side effects. Your doctor may reduce your dose, interrupt your treatment for a short period, or stop treatment completely if:
- you have a cough, shortness of breath, or swelling in your legs or arms (fluid retention) – these may be signs of heart problems (congestive heart failure)
- you feel confused, mood changes, memory problems, or see things that are not there (hallucinations)
- you feel dizzy or faint or feel that your heart is beating irregularly or fast – this may be a symptom of an abnormal heartbeat
- you notice any joint pain, bone pain, deformities, or changes in your ability to move, as this may be a sign of fractures
- you have kidney problems or arthritis, – you may have high uric acid in the blood
Tell your doctor immediately if you notice any of the above side effects.
Other side effects
Tell your doctor, pharmacist, or nurse if you experience any of the following side effects:
Very common:may affect more than 1 in 10 people:
- fatigue
- taste alteration
- feeling unsteady or dizzy
- blurred vision
- swelling
- diarrhea or constipation
- feeling or being sick
- difficulty swallowing
- abnormal sensation, numbness, tingling, pricking, or burning sensation
- skin rash
- difficulty breathing
- cough or fever
- headache
- weight gain
- vomiting
- muscle pain or weakness
- pain, including back, neck, musculoskeletal, or limb pain
- stomach pain
- joint pain
- unpleasant abnormal sensation in the arms or legs
- loss of muscle control, unsteadiness when walking
- alteration in normal sleep patterns
- lung infection
- urinary tract infection
- inability to empty the bladder completely
- loss of appetite
- low blood pressure
- decrease in the number of a type of white blood cell called neutrophils
- decrease in the number of red blood cells (anemia)
- increase in the levels of certain liver enzymes (AST/ALT) in the blood
- increase in the levels of creatinine in the blood (a substance that is normally removed in the urine through the kidneys)
Common:may affect up to 1 in 10 people:
- mood changes
- dehydration
- fluid in the lungs
- fainting
- sensitivity of the skin to sunlight
Uncommon:may affect less than 1 in 100 people:
- changes in certain chemical components in your blood, due to the rapid breakdown of tumor cells – this may cause damage to organs, including kidneys, heart, and liver.
- Inflammation of the heart muscle.
Tell your doctor, pharmacist, or nurse if you experience any of the above side effects.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible that they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V*. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Rozlytrek
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the carton and blister after EXP. The expiry date refers to the last day of the month shown.
- Store the capsules in the original package and keep the bottle tightly closed to protect from moisture.
- After preparation of the oral suspension, store below 30°C and use within 2 hours of preparation.
- Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Content and Additional Information
Rozlytrek Composition
The active ingredient is entrectinib. Each sachet contains 50 mg of entrectinib.
The other components are:
- Granule core:microcrystalline cellulose (E460), tartaric acid (E334), anhydrous colloidal silica (E551), sodium croscarmellose (E468), sodium stearyl fumarate, mannitol (E421), magnesium stearate (E470b).
- Coating:titanium dioxide (E-171), talc, yellow iron oxide (E172), red iron oxide (E172), black iron oxide (E172), polyethylene glycol 3350, partially hydrolyzed polyvinyl alcohol.
Appearance of Rozlytrek and Container Content
Rozlytrek 50 mg film-coated granules are orange-brown or grayish-orange in color and are contained in a sachet. Each container contains 42 sachets.
Marketing Authorization Holder
Roche Registration GmbH
Emil-Barell-Strasse 1
79639 Grenzach-Wyhlen
Germany
Manufacturer
Roche Pharma AG
Emil-Barell-Strasse 1
79639 Grenzach-Wyhlen
Germany
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium/Luxembourg N.V. Roche S.A. Belgium Tel: +32 (0) 2 525 82 11 | Latvia Roche Latvija SIA Tel: +371 - 6 7039831 |
| Lithuania UAB “Roche Lietuva” Tel: +370 5 2546799 |
Czech Republic Roche s. r. O. Tel: +420 - 2 20382111 | Hungary Roche (Hungary) Kft. Tel: +36 1 279 4500 |
Denmark Roche Pharma A/S Tlf: +45 - 36 39 99 99 | Netherlands Roche Nederland B.V. Tel: +31 (0) 348 438050 |
Germany Roche Pharma AG Tel: +49 (0) 7624 140 | Norway Roche Norge AS Tlf: +47 - 22 78 90 00 |
Estonia Roche Eesti OÜ Tel: + 372 - 6 177 380 | Austria Roche Austria GmbH Tel: +43 (0) 1 27739 |
Greece/Cyprus Roche (Hellas) A.E. Greece Tel: +30 210 61 66 100 | Poland Roche Polska Sp.z o.o. Tel: +48 - 22 345 18 88 |
Spain Roche Farma S.A. Tel: +34 - 91 324 81 00 | Portugal Roche Farmacêutica Química, Lda Tel: +351 - 21 425 70 00 |
France Roche Tél: +33 (0) 1 47 61 40 00 | Romania Roche România S.R.L. Tel: +40 21 206 47 01 |
Croatia Roche d.o.o. Tel: +385 1 4722 333 | Slovenia Roche farmacevtska družba d.o.o. Tel: +386 - 1 360 26 00 |
Slovak Republic Roche Slovensko, s.r.o. Tel: +421 - 2 52638201 | |
Ireland, Malta Roche Products (Ireland) Ltd. Ireland Tel: +353 (0) 1 469 0700 | |
Iceland Roche Pharmaceuticals A/S c/o Icepharma hf Tel: +354 540 8000 | Finland Roche Oy Tel: +358 (0) 10 554 500 |
Italy Roche S.p.A. Tel: +39 - 039 2471 | Sweden Roche AB Tel: +46 (0) 8 726 1200 |
Date of Last Revision of this Leaflet
This medicinal product has been authorized with a “conditional approval”. This type of approval means that more information on this medicinal product is expected.
The European Medicines Agency will review the new information on this medicinal product at least once a year and this leaflet will be updated as necessary.
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu,
- Instructions for Use
Rozlytrek
(entrectinib)
film-coated granules, for oral use
These Instructions for Use contain information on how to prepare, take, and administer Rozlytrek film-coated granules.
Before Starting |
- Read these Instructions for Usebefore taking or administering Rozlytrek film-coated granules.
- Ask your doctor to show you how to use Rozlytrek before starting treatment.
- If you have any questions about the use of Rozlytrek, ask your doctor.
Important Information You Need to Know Before Preparing, Taking, or Administering Rozlytrek |
- Your doctor should teach you how to prepare and take or administer Rozlytrek film-coated granules correctly. Follow your doctor's instructions for administering Rozlytrek film-coated granules exactly.
- Do nottake or administer Rozlytrek to another person until you have been shown how to prepare and take or administer Rozlytrek correctly.
- Wash your hands before and after using Rozlytrek.
- Check the expiration date and for damage to the product before using it. Do notuse it if it has expired or is damaged.
- If you immediately suffer from vomiting or partial or total regurgitation after administering a dose, consult your doctor or pharmacist for further instructions.
- Administer it within 2 hours of preparation.
Administration of Rozlytrek film-coated granules |
Your doctor will decide the correct daily dose of Rozlytrek for you or your child.
- Each sachet contains 50 mg of Rozlytrek.
- Do notdivide the contents of a sachet of film-coated granules to prepare a smaller dose.
- Take Rozlytrek film-coated granules orally - sprinkled over soft food. The film-coated granules should be sprinkled over one or more tablespoons of soft food (such as applesauce, yogurt, or pudding) and taken within 20 minutes of preparation.
- Do not crush or chew the film-coated granules to avoid the bitter taste.
- Drink water after taking the medication.
- The film-coated granules must notbe used with a feeding tube - it could clog the tube.
Preparation for Administering Rozlytrek |
Step 1.Wash your hands. | |
Step 2.To administer a dose, you will need:
| |
Step 3.Count the number of sachets (50 mg each) needed to administer the prescribed dose (Figure A). |
|
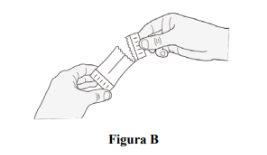
Step 4.Gently tap the sachet to ensure the film-coated granules are on one side of the sachet. Hold the side of the sachet where you tapped the granules and open the sachet with your hands or with scissors (Figure B). Note:Be careful not to cut the film-coated granules with the scissors. |
|
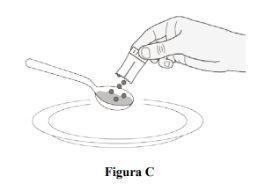
Step 5.Take a tablespoon of soft food and hold it over a paper towel or a clean plate. Sprinkle the prescribed number of sachets over the tablespoon of soft food (Figure C). Tap the sachets to ensure all the film-coated granules are sprinkled over the food. Note:You may need more than one tablespoon of soft food to administer the prescribed dose. |
|
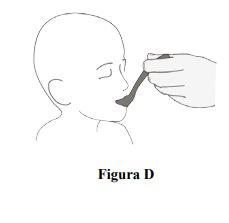
Step 6.Take or administer a tablespoon of food with the granules sprinkled on it immediately (Figure D). Take or administer the tablespoon of soft food within 20 minutes after sprinkling the film-coated granules if you cannot take it or administer it immediately. Note: Do notcrush or chew to avoid the bitter taste. If you leave the film-coated granules in the soft food for too long, the coating may dissolve and cause a bitter taste. If you do not take it within 20 minutes, discard the soft food with the film-coated granules sprinkled on it and prepare a new dose (start at Step 2). |
|
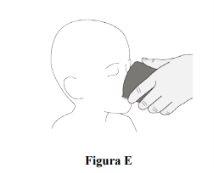
Step 7.Give patients a little water after administering Rozlytrek to ensure all the film-coated granules are swallowed (Figure E). Patients can be given any food or drink of their choice after administering Rozlytrek to improve the taste. |
|
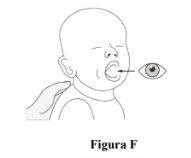
Step 8.Check the mouth to ensure all the film-coated granules have been swallowed correctly (Figure F). If not all the film-coated granules have been swallowed, administer a little water. |
|
Step 9.Wash your hands and wash the materials used to administer Rozlytrek. Dispose of disposable materials according to local requirements. |
Storage of Rozlytrek |
- Store below 30 °C in the original package of the film-coated granules to protect from moisture.
- Discard Rozlytrek if it is exposed to temperatures above 30 °C and follow the instructions for disposal indicated in step C1 and in section 5 of the leaflet.
- Always keep Rozlytrek out of sight and reach of children.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ROZLYTREK 50 mg FILM-COATED GRANULES IN SACHETDosage form: CAPSULE, 100 mgActive substance: entrectinibManufacturer: Roche Registration GmbhPrescription requiredDosage form: CAPSULE, 200 mgActive substance: entrectinibManufacturer: Roche Registration GmbhPrescription requiredDosage form: TABLET, 100 mgActive substance: avapritinibManufacturer: Blueprint Medicines (Netherlands) B.V.Prescription required
Online doctors for ROZLYTREK 50 mg FILM-COATED GRANULES IN SACHET
Discuss questions about ROZLYTREK 50 mg FILM-COATED GRANULES IN SACHET, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions