ROZLYTREK 200 mg HARD CAPSULES

How to use ROZLYTREK 200 mg HARD CAPSULES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Rozlytrek 100 mg Hard Capsules
Rozlytrek 200 mg Hard Capsules
entrectinib
This medicine is subject to additional monitoring. This will help to quickly identify new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects. Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
- This leaflet has been written in a way that is easy for most people to understand. However, if you are not familiar with medical terms, you should ask your doctor or pharmacist to explain anything you do not understand.
If you are giving this medicine to your child, replace "you" with "your child" throughout this leaflet.
Contents of the pack
- What is Rozlytrek and what is it used for
- What you need to know before you take Rozlytrek
- How to take Rozlytrek
- Possible side effects
- How to store Rozlytrek
- Contents of the pack and other information
- Instructions for use
1. What is Rozlytrek and what is it used for
What is Rozlytrek
Rozlytrek is a cancer medicine that contains the active substance entrectinib.
What is Rozlytrek used for
Rozlytrek is used to treat:
- adults, adolescents, and children over 1 month of age with solid tumors (cancer) in various parts of the body, which are caused by a change in the gene called neurotrophic tyrosine receptor kinase (NTRK)
- adults with a type of lung cancer called "non-small cell lung cancer" (NSCLC), which is caused by a change in the ROS1gene.
This medicine is used for solid tumors when:
- a test has shown that your cancer cells have a change in the NTRKgenes (see "How Rozlytrek works" below), and
- your cancer has spread within the affected organ or to other organs in your body, or if surgery to remove the cancer is likely to cause serious complications, and
- you have not received previous treatment with NTRKinhibitor medicines
- other treatments have not been effective or are not suitable for you.
This medicine is used for non-small cell lung cancer (NSCLC):
- it is ROS1-positive: this means that your cancer cells have a change in a gene called ROS1(see "How Rozlytrek works" below),
- it is advanced - for example, it has spread to other parts of your body (i.e., it is metastatic), and
- you have not received previous treatment with ROS1inhibitor medicines.
How Rozlytrek works
Rozlytrek works by blocking the action of faulty proteins. These faulty proteins are caused by a change in the genes that produce them, called NTRKor ROS1. These faulty proteins stimulate the growth of cancer cells.
Rozlytrek may slow down or stop the growth of the cancer. It may also help to reduce it.
2. What you need to know before you take Rozlytrek
Do not take Rozlytrek
- if you are allergic to entrectinib or any of the other ingredients of this medicine (listed in section 6).
If you are not sure, talk to your doctor, pharmacist, or nurse before taking Rozlytrek.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before taking Rozlytrek if:
- you have recently had memory loss, confusion, hallucinations, or changes in your mental state
- you have had bone fractures or conditions that may increase the risk of bone fractures, called "osteoporosis" or "osteopenia"
- you are taking medication to lower uric acid levels in your blood
- you have heart failure (when your heart has difficulty pumping blood to supply oxygen to the rest of your body) - the signs may include cough, difficulty breathing, and swelling in your legs and arms.
- you have ever had heart problems or electrical conduction problems in your heart called "prolonged QTc interval" - this is shown on an electrocardiogram (ECG) or by low levels of electrolytes in your blood.
- you have a hereditary problem called "galactose intolerance", "congenital lactase deficiency", or "glucose-galactose malabsorption"
If you have any of the above conditions (or are not sure), talk to your doctor or pharmacist before taking Rozlytrek.
Other medicines and Rozlytrek
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. This is because Rozlytrek may affect how other medicines work. Similarly, other medicines may affect how Rozlytrek works.
In particular, tell your doctor or pharmacist if you are taking any of the following medicines:
- for fungal infections (antifungals) - such as ketoconazole, itraconazole, voriconazole, posaconazole
- for HIV/AIDS - such as ritonavir or saquinavir
- for depression - such as paroxetine, fluvoxamine, or herbal remedies for depression - St. John's Wort
- to prevent seizures or fits - such as phenytoin, carbamazepine, or phenobarbital
- for tuberculosis - such as rifampicin, rifabutin
- for solid tumors and blood cancers - topotecan, lapatinib, mitoxantrone, apalutamide, or methotrexate
- for joint inflammation or autoimmune diseases of the joints (rheumatoid arthritis) - methotrexate
- for migraines - ergotamine
- for severe pain - fentanyl
- for mental illnesses (psychosis) or Tourette's syndrome - pimozide
- for irregular heartbeat - quinidine
- to prevent blood clots - warfarin or dabigatran etexilate
- for acid reflux (heartburn) - cisapride or omeprazole
- to lower cholesterol levels in your blood - atorvastatin, pravastatin, or rosuvastatin
- to suppress your immune system or prevent your body from rejecting a transplanted organ - sirolimus, tacrolimus, or cyclosporin
- to lower blood sugar levels - repaglinide or tolbutamide
- for high blood pressure - bosentan, felodipine, nifedipine, or verapamil
- for inflammation or nausea - dexamethasone
If you have any of the above conditions (or are not sure), talk to your doctor or pharmacist before taking Rozlytrek.
Using Rozlytrek with food and drink
Do not drink grapefruit juice or eat grapefruit or bitter oranges while taking this medicine. This can increase the amount of the medicine in your blood to harmful levels.
Pregnancy, breast-feeding, and fertility
Women and contraceptive measures
You must avoid becoming pregnant while taking this medicine, as it may harm the unborn baby. If you can become pregnant, you must use a highly effective method of contraception:
- during treatment, and
- for at least 5 weeks after stopping treatment.
Rozlytrek may reduce the effect of contraceptive medicines (birth control pills or hormonal contraceptives). You should use another reliable method of contraception, such as a barrier method (e.g., condoms).
Talk to your doctor about the contraceptive methods that are suitable for you and your partner.
Men and contraceptive measures
Your partner must avoid becoming pregnant while you are taking this medicine, as it may harm the unborn baby. If your partner can become pregnant, you must use a highly effective method of contraception:
- during treatment, and
- for at least 3 months after stopping treatment.
Talk to your doctor about the contraceptive methods that are suitable for you and your partner.
Pregnancy
- Do not take Rozlytrek if you are pregnant, as it may harm the unborn baby.
- If you become pregnant while taking this medicine or in the 5 weeks after your last dose, tell your doctor immediately.
Breast-feeding
Do not breast-feed while taking this medicine. It is not known whether Rozlytrek passes into breast milk, and it may harm your baby.
Driving and using machines
Rozlytrek may affect your ability to drive, ride a bicycle, or use machines. The following side effects may occur while taking Rozlytrek:
- blurred vision
- feeling tired, dizzy, or faint
- changes in your mental state, confusion, or seeing things that are not there (hallucinations).
If this happens, do not drive, ride a bicycle, or use heavy machinery until you feel better. Ask your doctor or pharmacist if you can drive, ride a bicycle, or use machines.
Rozlytrek contains
- Lactose- a type of sugar. If your doctor has told you that you have an intolerance to some sugars, contact them before taking this medicine.
- Orange Yellow FCF (E-110) only in 200 mg hard capsules.This is a coloring agent that may cause allergic reactions.
3. How to take Rozlytrek
Follow exactly the instructions given to you by your doctor or pharmacist. If you are not sure, talk to your doctor or pharmacist again.
How much to take
Adults
- The recommended dose is 3 capsules of 200 mg once a day (this is equivalent to a total dose of 600 mg).
- If you feel unwell, your doctor may reduce your dose, interrupt your treatment for a short period, or stop your treatment completely.
Adolescents and children over 1 month of age:
Your doctor will calculate the correct dose for you based on your height and weight.
Your doctor will review your dose and change it as necessary.
Rozlytrek is also available as a film-coated granule in a sachet for patients who cannot swallow capsules but can swallow soft foods.
How to take it
Rozlytrek can be taken with or without food.
There are two ways your doctor may tell you to take Rozlytrek capsules:
- Swallow each capsule whole. Do not crush or chew the capsules.
- Take it prepared as an oral suspension (using a syringe) or, if necessary, through a feeding tube.
Read the "Instructions for Use" at the end of the leaflet.
Read and follow carefully the "Instructions for Use" at the end of the leaflet on how to take and administer Rozlytrek. Detailed information is shown on how to prepare, measure, take, or administer Rozlytrek prepared as an oral suspension:
- by mouth, or
- through a feeding tube (such as a gastric or nasogastric tube).
If you vomit after taking Rozlytrek
Capsules
If you vomit immediately after taking a dose of Rozlytrek, take another dose.
Capsules administered as an oral suspension
If you vomit or have partial regurgitation immediately after taking the dose, talk to your doctor or pharmacist about what to do.
If you take more Rozlytrek than you should
If you take more Rozlytrek than you should, talk to a doctor or go to the hospital immediately. Take the medicine pack and this leaflet with you.
If you forget to take Rozlytrek
- If it is more than 12 hours until your next dose, take the missed dose as soon as you remember.
- If it is less than 12 hours until your next dose, do not take the missed dose. Take your next dose at the usual time.
- Do not take a double dose to make up for a forgotten dose.
If you stop taking Rozlytrek
Do not stop taking this medicine without talking to your doctor first. It is important that you take this medicine every day for as long as your doctor has prescribed it.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The following side effects may happen with this medicine.
Serious side effects
Tell your doctor immediately if you experience any of the following serious side effects. Your doctor may reduce your dose, interrupt your treatment for a short period, or stop your treatment completely if:
- you have a cough, difficulty breathing, or swelling in your legs or arms (fluid retention) - these may be signs of heart problems (congestive heart failure)
- you feel confused, changes in mood, memory problems, or see things that are not there (hallucinations)
- you feel dizzy or lightheaded or feel that your heart is beating irregularly and quickly - this may be a symptom of an abnormal heartbeat
- you notice any joint pain, bone pain, deformities, or changes in your ability to move, as this may be a sign of fractures
- you have kidney problems or arthritis - you may have high levels of uric acid in your blood
Tell your doctor immediately if you notice any of the above side effects.
Other side effectsTell your doctor, pharmacist, or nurse if you experience any of the following side effects:
Very common:may affect more than 1 in 10 people:
- fatigue
- altered taste
- feeling unsteady or dizzy
- blurred vision
- swelling
- diarrhea or constipation
- feeling or being sick
- difficulty swallowing
- abnormal sensation, numbness, tingling, or burning sensation
- rash
- difficulty breathing
- cough or fever
- headache
- weight gain
- vomiting
- muscle pain or weakness
- pain, including back, neck, musculoskeletal, or limb pain
- stomach pain
- joint pain
- unpleasant abnormal sensation in the arms or legs
- loss of muscle control, unsteadiness when walking
- changes in normal sleep patterns
- lung infection
- urinary tract infection
- inability to empty the bladder completely
- loss of appetite
- low blood pressure
- decrease in the number of a type of white blood cell called neutrophils
- decrease in the number of red blood cells (anemia)
- increase in the levels of certain liver enzymes (AST/ALT) in the blood
- increase in the levels of creatinine in the blood (a substance that is normally removed in the urine through the kidneys)
Common:may affect up to 1 in 10 people:
- mood changes
- dehydration
- fluid in the lungs
- fainting
- sensitivity of the skin to sunlight
Uncommon:may affect up to 1 in 100 people:
Changes in certain chemical components in your blood due to the rapid breakdown of tumor cells - this may cause damage to organs, including kidneys, heart, and liver.
- inflammation of the heart muscle.
Tell your doctor, pharmacist, or nurse if you experience any of the above side effects.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Rozlytrek
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the carton and blister after EXP. The expiry date refers to the last day of the month shown.
- Store the capsules in the original package and keep the bottle tightly closed to protect from moisture.
- After preparation of the oral suspension, store below 30°C and use within 2 hours of preparation.
- Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Rozlytrek Composition
The active ingredient is entrectinib.
Rozlytrek 100 mg: each capsule contains 100 mg of entrectinib. Rozlytrek 200 mg: each capsule contains 200 mg of entrectinib.
The other ingredients are:
- Capsule Content:tartaric acid (E334), lactose (see section 2 "Rozlytrek contains lactose"), hypromellose (E464), crospovidone (E1202), microcrystalline cellulose (E460), anhydrous colloidal silica (E551), magnesium stearate (E470b).
- Capsule Shell:hypromellose (E464), titanium dioxide (E-171), yellow iron oxide (E172; for Rozlytrek 100 mg capsule), orange yellow FCF (E-110; for Rozlytrek 200 mg capsule. See section 2 "Rozlytrek contains orange yellow FCF (E110)").
- Printing Ink:shellac, propylene glycol, aluminum lake indigo carmine (E-132).
Appearance of Rozlytrek and Container ContentsRozlytrek 100 mg hard opaque yellow capsules with ENT 100 printed in blue ink on the body.
Rozlytrek 200 mg hard opaque orange capsules with ENT 200 printed in blue ink on the body.
The capsules are presented in bottles containing:
- 30 hard capsules of Rozlytrek 100 mg, or
- 90 hard capsules of Rozlytrek 200 mg.
Marketing Authorization Holder
Roche Registration GmbH
Emil-Barell-Strasse 1
79639 Grenzach-Wyhlen
Germany
Manufacturer
Roche Pharma AG
Emil-Barell-Strasse 1
79639 Grenzach-Wyhlen
Germany
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:

|
Denmark Roche Pharmaceuticals A/S Tlf: +45 - 36 39 99 99 | Netherlands Roche Nederland B.V. Tel: +31 (0) 348 438050 |
Germany Roche Pharma AG Tel: +49 (0) 7624 140 | Norway Roche Norge AS Tlf: +47 - 22 78 90 00 |
Estonia Roche Eesti OÜ Tel: + 372 - 6 177 380 | Austria Roche Austria GmbH Tel: +43 (0) 1 27739 |
Greece Roche (Hellas) A.E. Τηλ: +30 210 61 66 100 | Poland Roche Polska Sp.z o.o. Tel: +48 - 22 345 18 88 |
Spain Roche Farma S.A. Tel: +34 - 91 324 81 00 | Portugal Roche Farmacêutica Química, Lda Tel: +351 - 21 425 70 00 |
France Roche Tél: +33 (0) 1 47 61 40 00 | Romania Roche România S.R.L. Tel: +40 21 206 47 01 |
Croatia Roche d.o.o. Tel: +385 1 4722 333 Ireland Roche Products (Ireland) Ltd. Tel: +353 (0) 1 469 0700 | Slovenia Roche farmacevtska družba d.o.o. Tel: +386 - 1 360 26 00 |
Iceland Roche Pharmaceuticals A/S c/o Icepharma hf Sími: +354 540 8000 | Slovak Republic Roche Slovensko, s.r.o. Tel: +421 - 2 52638201 |
Italy Roche S.p.A. Tel: +39 - 039 2471 | Finland Roche Oy Puh/Tel: +358 (0) 10 554 500 |
Sweden Roche AB Tel: +46 (0) 8 726 1200 | |
Date of Last Revision of this Prospectus
This medicinal product has been authorized with a "conditional approval". This type of approval means that more information is expected to be obtained for this medicinal product.
The European Medicines Agency will review the new information for this medicinal product at least once a year, and this prospectus will be updated as necessary.
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu,
<------------------------------------------------------------------------------------------------------------------------------->
- Instructions for Use
Rozlytrek
(entrectinib)
Capsules for oral use
(administered as a whole capsule or as an oral suspension)
These instructions for use contain information on how to prepare, take, and administer Rozlytrek capsules.
Rozlytrek capsules can be swallowed whole or prepared as a suspension (in water or milk) and administered orally or through a gastric or nasogastric tube.
Before Starting
- Read these Instructions for Usebefore taking or administering Rozlytrek capsules.
- Ask your doctor to show you how to use Rozlytrek before starting treatment.
- If you have any questions about using Rozlytrek, ask your doctor.
Important Information You Need to Know Before Preparing, Taking, or Administering Rozlytrek
- Your doctor should teach you how to prepare and take or administer Rozlytrek capsules correctly. Follow your doctor's instructions for administering Rozlytrek exactly.
- Do not take oradminister Rozlytrek to another person until you have been shown how to prepare and take or administer Rozlytrek correctly.
- Wash your hands before and after using Rozlytrek. Do nottouch your eyes, nose, or mouth during the preparation of the oral suspension.
- Check the expiration date and for any damage to the product before using it. Do notuse it if it has expired or is damaged.
- For whole capsules, if you vomit immediately after taking the dose of Rozlytrek, take another dose.
- For capsules administered as an oral suspension, if you vomit or have partial or total regurgitation immediately after administering a dose, consult your doctor or pharmacist for further instructions.
- The oral suspension should be administered within 2 hours of preparation.
Administration of Rozlytrek Capsules Orally
Your doctor will decide the correct daily dose of Rozlytrek for you or your child.
- Swallow the capsules whole, with or without food, with a little water, as directed by your doctor.
- Do not crush or chew the capsules.
Administration of Rozlytrek as a Liquid Suspension – Orally or Through a Gastric/Nasogastric Tube
If you or your child cannot swallow whole capsules, Rozlytrek capsules can be prepared as a suspension (in water or milk) and administered orally or through a feeding tube.
Your doctor will tell you the number of capsules to use, the exact amount of liquid (water or milk) to mix with the contents of the capsule(s) needed to prepare the suspension, and the exact amount (ml) of suspension to extract to reach the prescribed dose of Rozlytrek to take or administer.
In Table 1, the prescribed dose, the number and concentration of capsules needed, the amount of water or milk to mix with the contents of the capsule(s) to prepare the suspension, and the amount of suspension to extract to reach the prescribed dose to take or administer are shown.
It is possible that you will need to measure a smaller amount of suspension than you prepared to take or administer the correct prescribed dose of Rozlytrek.
Table 1. Preparation of Rozlytrek Capsules as a Suspension | |||
Prescribed Dose of Rozlytrek to Administer | Number of 100 mg or 200 mg Capsules Needed | Amount of Water or Milk to Mix with the Contents of the Capsule(s) for Suspension Preparation | Amount of Suspension to Extract to Reach the Prescribed Dose |
20 mg | One 100 mg | 5 ml | 1 ml |
30 mg | One 100 mg | 5 ml | 1.5 ml |
40 mg | One 100 mg | 5 ml | 2 ml |
50 mg | One 100 mg | 5 ml | 2.5 ml |
60 mg | One 100 mg | 5 ml | 3 ml |
70 mg | One 100 mg | 5 ml | 3.5 ml |
80 mg | One 100 mg | 5 ml | 4 ml |
90 mg | One 100 mg | 5 ml | 4.5 ml |
100 mg | One 100 mg | 5 ml | 5 ml |
110 mg | One 200 mg | 10 ml | 5.5 ml |
120 mg | One 200 mg | 10 ml | 6 ml |
130 mg | One 200 mg | 10 ml | 6.5 ml |
140 mg | One 200 mg | 10 ml | 7 ml |
150 mg | One 200 mg | 10 ml | 7.5 ml |
200 mg | One 200 mg | 10 ml | 10 ml |
300 mg | Three 100 mg | 15 ml | 15 ml |
400 mg | Two 200 mg | 20 ml | 20 ml |
600 mg | Three 200 mg | 30 ml | 30 ml |
To prepare the suspension, you will need:
- The number of capsules indicated by your doctor
- A clean, empty glass (not included in the packaging)
- A glass of drinking water or milk at room temperature (below 30°C)
- An oral syringe (provided by your pharmacist) with 0.5 ml gradations
- A paper towel
Preparation of Rozlytrek Suspension
Step 1.Wash your hands. | |
Step 2.Count the number of capsules indicated by your doctor to prepare the suspension. | |
Step 3.Place a clean, empty glass on a paper towel. |
Figure A |
Step 4.Gently tap the capsule to empty its contents. | |
Step 5.Hold the capsule over the clean, empty glass to avoid spills. | |
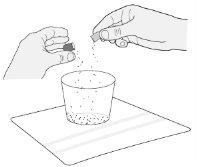
Step 6.Open the capsule by gently pressing and twisting both sides. Pour the contents into the clean glass (Figure A). | |
Step 7.Gently tap both sides of the capsule and check that all the contents have fallen into the glass. If the capsule contents spill outside the glass, empty the glass contents and use another capsule. Go to Step C1 for cleaning instructions and start again at Step 1. | |
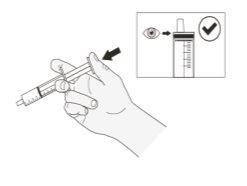
Step 8.Push the syringe plunger to the bottom to remove air from the syringe (Figure B). |
Figure B |
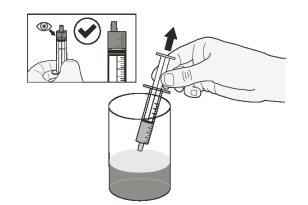
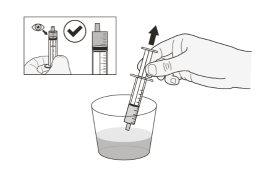
Step 9.Take the glass of water or milk at room temperature (below 30°C). Using the syringe, draw up the exact volume of drinking water or milk at room temperature from the glass (Figure C). *Your doctor will tell you the amount of liquid to use. Do notuse any other type of liquid. |
Figure C |
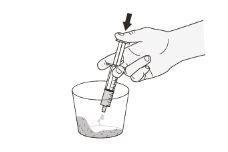
Step 10.Add the water or milk from the syringe to the glass containing the capsule(s) contents (Figure D). |
Figure D |
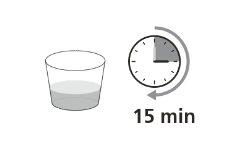
Step 11.Let the suspension stand for 15 minutes (Figure E). Note:This is important to achieve a uniform suspension; otherwise, you may not get the correct dose. |
Figure E |
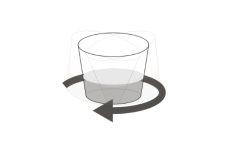
Step 12.Stir the suspension several times to mix the medicine uniformly with the liquid (Figure F). Note:The suspension will appear cloudy if water is used. |
Figure F |
Step 13.Push the syringe plunger to the bottom to remove air from the syringe (Figure G). |
Figure G |
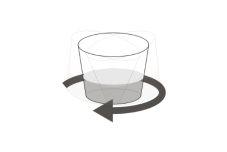
Step 14.Turn the glass again before placing the syringe in the glass (Figure H). |
Figure H |
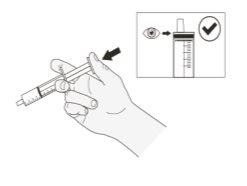
Step 15.Immediately place the syringe in the glass and slowly pull the plunger back to draw up the exact amount of suspension to reach the prescribed dose of Rozlytrek (Figure I).
|
Figure I |
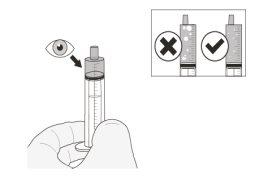
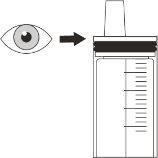
Step 16.Check the amount in the syringe (Figure J). With the syringe tip pointing upwards, check that:
Note:If you have not drawn up the correct volume or if there are large bubbles inside:
Shake the syringe quickly. Administer Rozlytrek immediately after drawing it up with the syringe. If not taken within 2 hours, discard the medicine from the syringe. Go to Step C1 for cleaning instructions and start again at Step 2 to mix a new dose. |
Figure J |
Oral Administration
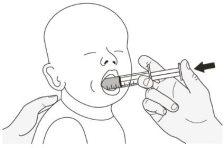
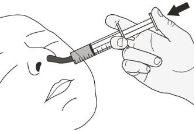
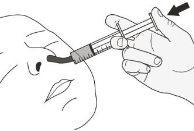
Step A1.Have the patient lie on their back when administering a dose of Rozlytrek orally (Figure K). Place the syringe in the mouth, with the tip along the cheek. Slowly push the plunger to the bottom. Note:If Rozlytrek is administered too quickly, choking may occur. |
Figure K |
Step A2.Check that no medicine remains in the syringe (Figure L). If suspension remains in the syringe, repeat Step A1. |
Figure L |
Step A3.Drink a little water immediately after administering the prescribed dose of Rozlytrek. In case of a bad taste, you can breastfeed the child or give them milk. |
Administration Through a Gastric or Nasogastric Tube
You can take or administer the suspension through a nasogastric or gastric tube placed by a healthcare professional. Check the manufacturer's instructions for the size and dimensions of the enteral zone. Ensure the tube size is at least 8 French or larger to avoid tube obstruction if the aliquots (amount of suspension) are 3 ml or more. To take or administer Rozlytrek in doses of 3 ml or more, divide the dose and give it in at least 2 parts. Flush the tube with the same amount of water or milk after administering each part. Neonates and children with fluid restrictions may require minimum flush volumes of 1 to 3 ml to administer Rozlytrek. The aliquots should be adjusted accordingly. To take or administer 30 ml doses of Rozlytrek, divide the dose into at least 3 parts of 10 ml. Flush the tube with the same amount of water or milk. |
Of water or milk after administering each part. The tube should be flushed with water or milk after administering Rozlytrek. If you have any doubts, consult your doctor. | ||
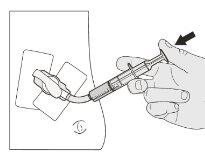
Step B1 Place the tip of the syringe into the nasogastric/gastric tube. Slowly press the plunger to the bottom to administer the full dose of Rozlytrek (Figure M1 and M2). |
Figure M1 |
Figure M2 |
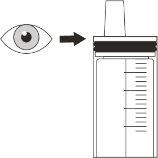
Step B2 Check that no medication remains in the syringe (Figure N). |
Figure N | |
Step B3 Flush the nasogastric/gastric tube with water or milk* immediately after administering the prescribed dose (Figure O1 and O2). *Your doctor will indicate the amount of water or milk to use for flushing. |
Figure O1 |
Figure O2 |
Step C1
|
Storage of Rozlytrek
- Keep below 30°C in the original packaging and keep the bottle tightly closed to protect it from moisture.
- Discard Rozlytrek if it is exposed to temperatures above 30°C and follow the instructions for disposal indicated in Step C1 and in Section 5 of the package insert.
- After preparing the oral suspension, keep below 30°C and use within 2 hours of preparation.
- Always keep Rozlytrek out of sight and reach of children.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ROZLYTREK 200 mg HARD CAPSULESDosage form: CAPSULE, 100 mgActive substance: entrectinibManufacturer: Roche Registration GmbhPrescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 50 mgActive substance: entrectinibManufacturer: Roche Registration GmbhPrescription requiredDosage form: TABLET, 100 mgActive substance: avapritinibManufacturer: Blueprint Medicines (Netherlands) B.V.Prescription required
Online doctors for ROZLYTREK 200 mg HARD CAPSULES
Discuss questions about ROZLYTREK 200 mg HARD CAPSULES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions